-

-

-
Peter Thomas
Professor Emeritus (Interim Appointment)
Department of Marine Science, Marine Science Institute
H-E-B Endowed Chair in Marine Science (Emeritus)Fish Reproductive Physiology, Marine Environmental Toxicology-
Download CV
Ph.D., Physiology, Department of Physiology, University of Leicester, Leicester, UK (1978)
B.Sc., Zoology with Psychology and Chemistry, University of Hull, Hull, UK (1970)
Research InterestsReproductive Endocrinology / Marine Environmental Toxicology
Endocrine control of reproduction in fishes and other vertebrates, rapid steroid hormone actions through novel steroid membrane receptors, applications of endocrinology in fish culture, effects of environmental factors such as hypoxia and pollutants on reproductive endocrine function.
A current research emphasis is on the structure, functions and evolution of a new class of sex steroid receptors we recently discovered in fishes and subsequently have identified in other vertebrates. The novel receptors are structurally unrelated to nuclear steroid receptors and are located on the surface of cells where they elicit rapid biological responses to steroids by mechanisms not involving gene transcription. Recent studies in a variety of vertebrates indicate these receptors mediate steroid regulation of meiosis in oocytes, sperm motility, gonadotropin-releasing hormone (GnRH) secretion and immune function.The release of nutrients and toxic chemicals into estuarine and coastal environments has increased dramatically in many parts of the world as a result of rapid population growth, industrialization and the adoption of intense agricultural practices, but the long-term ecological consequences of these changes are unknown. The development of early-warning biomarkers of environmental deterioration may enable environmental deterioration to be detected early enough for remedial actions can be taken. Our research emphasis has been on the development of endocrine biomarkers of impaired reproductive function in marine fish because a wide range of environmental stressors can disrupt fish reproduction with potential deleterious effects on the long-term maintenance of fish populations.
-
Fish Reproductive Physiology / Marine Environmental Toxicology Overview
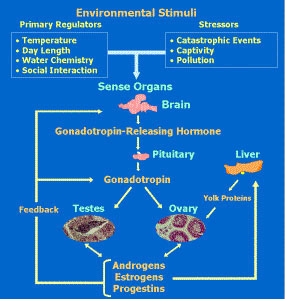 Schematic representation of the endocrine system controlling reproduction in fishes. My broad research interest is the endocrine control of reproduction in vertebrates with an emphasis on teleost fishes and how adverse environmental conditions such as hypoxia and toxic chemicals affect their endocrine and reproductive cycles. Research is conducted primarily on zebrafish and marine fishes such as Atlantic croaker and southern flounder at the Marine Science Institute at Port Aransas, Texas, and also in the northern Gulf of Mexico hypoxic zone. Current research includes the endocrine control of gamete maturation and the mechanisms of hypoxia disruption of reproduction, with an emphasis on the neuroendocrine system. A primary research focus is on rapid, nongenomic steroid actions mediated by novel membrane receptors which were first discovered by our research group in fish. Subsequently we identified these receptors in mammals and currently several projects are being conducted in human and cancer cell models on their characteristics and roles in human diseases such as infertility, cancer development, preterm birth and hypertension.
Schematic representation of the endocrine system controlling reproduction in fishes. My broad research interest is the endocrine control of reproduction in vertebrates with an emphasis on teleost fishes and how adverse environmental conditions such as hypoxia and toxic chemicals affect their endocrine and reproductive cycles. Research is conducted primarily on zebrafish and marine fishes such as Atlantic croaker and southern flounder at the Marine Science Institute at Port Aransas, Texas, and also in the northern Gulf of Mexico hypoxic zone. Current research includes the endocrine control of gamete maturation and the mechanisms of hypoxia disruption of reproduction, with an emphasis on the neuroendocrine system. A primary research focus is on rapid, nongenomic steroid actions mediated by novel membrane receptors which were first discovered by our research group in fish. Subsequently we identified these receptors in mammals and currently several projects are being conducted in human and cancer cell models on their characteristics and roles in human diseases such as infertility, cancer development, preterm birth and hypertension.CORE RESEARCH AREAS
Steroid Hormone Receptors: Genomic and Nongenomic Mechanisms
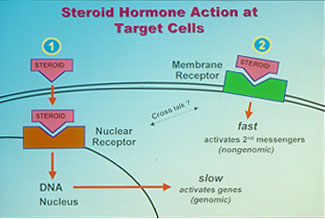 Fig. 1: Receptor-mediated mechanisms of steroid hormone action. Steroid hormones and their receptors are of central importance in the endocrine control of a wide variety of physiological functions critical for the survival and propagation of a species, such as reproduction, behavior, salt balance, metabolism, immune function and resistance to stress. Steroids have been shown to influence many of these processes by freely diffusing into the cell and binding to specific intracellular receptors. Activation of these nuclear steroid receptors by the hormone enables them to bind to specific hormone response elements on genes, resulting in alterations in their rates of transcription and subsequently protein synthesis (fig 1.) This classical genomic mechanism of steroid action is typically slow. However, over the past twenty years, convincing evidence has been obtained from many laboratories using a wide variety of animal and cell models that steroids also exert rapid actions at the cell-surface which are often nongenomic and instead involve activation of intracellular second messenger pathways (fig 1). Moreover, specific steroid receptors have been identified on the plasma membranes of target tissues which are the likely mediators of these nongenomic steroid actions.
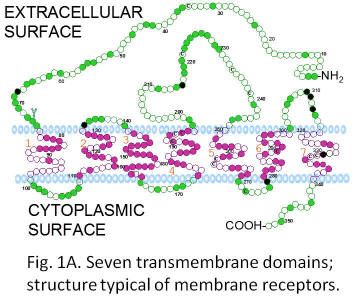
Fig. 1: Receptor-mediated mechanisms of steroid hormone action. Steroid hormones and their receptors are of central importance in the endocrine control of a wide variety of physiological functions critical for the survival and propagation of a species, such as reproduction, behavior, salt balance, metabolism, immune function and resistance to stress. Steroids have been shown to influence many of these processes by freely diffusing into the cell and binding to specific intracellular receptors. Activation of these nuclear steroid receptors by the hormone enables them to bind to specific hormone response elements on genes, resulting in alterations in their rates of transcription and subsequently protein synthesis (fig 1.) This classical genomic mechanism of steroid action is typically slow. However, over the past twenty years, convincing evidence has been obtained from many laboratories using a wide variety of animal and cell models that steroids also exert rapid actions at the cell-surface which are often nongenomic and instead involve activation of intracellular second messenger pathways (fig 1). Moreover, specific steroid receptors have been identified on the plasma membranes of target tissues which are the likely mediators of these nongenomic steroid actions.A decade ago, we discovered a novel gene in the ovaries of spotted seatrout that has all the characteristics of a membrane progesterone receptor and is important in egg and sperm maturation. The receptors have seven transmembrane domains which is a typical structure for membrane receptors (fig 1A). This is the first evidence for the existence of steroid receptors different from the intracellular steroid receptors. Subsequently, we found 13 closely related genes in other animals, including humans, that comprise an entirely new family of steroid receptors. These results were published in 2003 as back-to back papers in a leading scientific journal, Proceedings of the National Academy of Sciences, and caused great interest among the scientific community because they provided a plausible explanation for many of the unresolved issues in endocrinology and cancer biology.
 Over the past few years we have identified these membrane progesterone receptors (mPRs) on human sperm, where they appear to control motility and fertility, as well as throughout the female reproductive system. The progesterone membrane receptors are also present in the womb of pregnant women are likely involved in the induction of labor at term. The mPRs are also present in human ovarian and breast cancer cells and the amounts of the receptors are increased in human breast cancer tissues, suggesting that they are involved in the development and growth of the tumors. Current models on human diseases such as premature birth, hypertension and cancer are currently being studied. Drugs are being developed that can selectively target these receptors as potential therapeutic agents to treat human diseases. Fundamental research on how mPRs function to induce biological responses in cells is ongoing, both in fish and mammalian models which has been helped by collaborations with scientists throughout the world.
Over the past few years we have identified these membrane progesterone receptors (mPRs) on human sperm, where they appear to control motility and fertility, as well as throughout the female reproductive system. The progesterone membrane receptors are also present in the womb of pregnant women are likely involved in the induction of labor at term. The mPRs are also present in human ovarian and breast cancer cells and the amounts of the receptors are increased in human breast cancer tissues, suggesting that they are involved in the development and growth of the tumors. Current models on human diseases such as premature birth, hypertension and cancer are currently being studied. Drugs are being developed that can selectively target these receptors as potential therapeutic agents to treat human diseases. Fundamental research on how mPRs function to induce biological responses in cells is ongoing, both in fish and mammalian models which has been helped by collaborations with scientists throughout the world.
Several years later in 2005 we provided clear evidence that an orphan receptor in breast cancer cells, GPR30, has all the characteristics of an estrogen receptor. These finding were confirmed by another research group and has led to a flurry of research activity on its functions by research groups throughout the world. To date over 300 papers have been published on the biological role of GPR30. There is evidence for a role for GPR30 in insulin secretion, cardiovascular protection, development of cancer, neuroendocrine function, and other reproductive functions. We have also identified GPR30 in fish and have shown it has an important role in oocyte maturation, exerting an opposite effect to that of the mPRs, preventing precocious meiotic maturation. Currently we are exploring the basic characteristics of GPR30 in both mammalian and fish models.Recently, we identified a novel androgen receptor in fish ovaries that belong to the zinc transporter ZIP9 subfamily and are currently investigating its characteristics and biological functions in fish and human cancer cells.
Environmental Toxicology
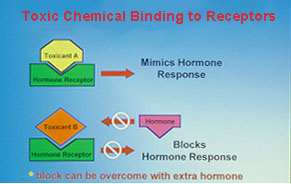 Fig. 2: Consequences of interactions of chemicals with hormone receptors.There is growing concern over recent reports of reproductive impairment and developmental abnormalities in fish and wildlife environmentally exposed to endocrine disrupting chemicals. Feminization of males birds, alligators and fishes, the production of the estrogen-induced yolk precursor, vitellogenin, in male fish, altered endocrine function in females and decreased reproductive success have been reported after exposure to a growing list of environmental contaminants including DDT, PCBs, pesticides, surfactants, plasticizers and heavy metals. It has been suggested that the increases in the incidence of breast and prostate cancers and decreased fertility and sperm counts in human populations are also due to environmental exposure to endocrine disrupting chemicals. However, our incomplete knowledge of the causes, consequences, and extent of endocrine disruption in vertebrates limits our ability to estimate the ecological and human health hazards of environmental exposure to endocrine disrupting chemicals.
Fig. 2: Consequences of interactions of chemicals with hormone receptors.There is growing concern over recent reports of reproductive impairment and developmental abnormalities in fish and wildlife environmentally exposed to endocrine disrupting chemicals. Feminization of males birds, alligators and fishes, the production of the estrogen-induced yolk precursor, vitellogenin, in male fish, altered endocrine function in females and decreased reproductive success have been reported after exposure to a growing list of environmental contaminants including DDT, PCBs, pesticides, surfactants, plasticizers and heavy metals. It has been suggested that the increases in the incidence of breast and prostate cancers and decreased fertility and sperm counts in human populations are also due to environmental exposure to endocrine disrupting chemicals. However, our incomplete knowledge of the causes, consequences, and extent of endocrine disruption in vertebrates limits our ability to estimate the ecological and human health hazards of environmental exposure to endocrine disrupting chemicals.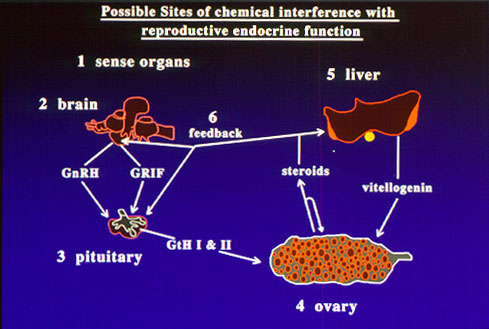 A major research emphasis in our laboratory has been to identify the mechanisms by which chemicals can interfere with reproductive endocrine function in the croaker and seatrout models. A wide variety of chemicals can disrupt chemical processes by binding to hormone receptors. If chemical binding causes activation of the receptor leading to a hormonal response, the chemical acts as a hormonal mimic. We and others have shown that o,p'-DDT, nonylphenol and hydroxylated PCBs act in this way on the nuclear estrogen receptor, causing an estrogenic response. On the other hand, if binding of the chemical to the receptor does not activate the receptor, no hormonal response occurs and the natural hormone cannot occupy the binding site, so the chemical acts as an antagonist (fig 2). A variety of organochlorine pesticides antagonize the actions of testosterone and progestins by this mechanism. Recently we have obtained the first clear evidence that these chemicals can also interfere with nongenomic steroid actions on oocytes, sperm and testes by binding to their steroid membrane receptors.
A major research emphasis in our laboratory has been to identify the mechanisms by which chemicals can interfere with reproductive endocrine function in the croaker and seatrout models. A wide variety of chemicals can disrupt chemical processes by binding to hormone receptors. If chemical binding causes activation of the receptor leading to a hormonal response, the chemical acts as a hormonal mimic. We and others have shown that o,p'-DDT, nonylphenol and hydroxylated PCBs act in this way on the nuclear estrogen receptor, causing an estrogenic response. On the other hand, if binding of the chemical to the receptor does not activate the receptor, no hormonal response occurs and the natural hormone cannot occupy the binding site, so the chemical acts as an antagonist (fig 2). A variety of organochlorine pesticides antagonize the actions of testosterone and progestins by this mechanism. Recently we have obtained the first clear evidence that these chemicals can also interfere with nongenomic steroid actions on oocytes, sperm and testes by binding to their steroid membrane receptors.
Studies are also being conducted on chemical disturbance of hormone signaling pathways at sites distal to binding of the hormone to its receptor. For example, we have obtained evidence that cadmium can disturb gonadotropin and estrogen secretion by by interfering with calcium signaling and the adenylate cyclase system, respectively.
Another major project is to determine the mechanisms of neuroendocrine toxicity by environmental chemicals. Many chemicals are known to be neurotoxic, but their actions on the neuroendocrine system and the reproductive consequences are largely unknown. Our studies have shown that PCBs disrupt reproductive cycles by inhibiting an enzyme involved in the synthesis of serotonin, a neurotransmitter which has a stimulatory influence on gonadotropin secretion. The results of this project, funded by the National Institutes of Health, have important implications for human health. Graduate student collecting croaker from the Dead Zone in the Gulf of Mexico.
Graduate student collecting croaker from the Dead Zone in the Gulf of Mexico.
 Croaker collected from a heavily contaminated site in the Houston Ship Channel.
Croaker collected from a heavily contaminated site in the Houston Ship Channel.The endocrine parameters altered in the controlled laboratory exposures have also been evaluated as biomarkers of exposure to endocrine disrupting chemicals in field studies in Texas, California and elsewhere. Currently, male croaker populations from contaminated and relatively clean sites in Texas bays and estuaries are being assessed for evidence of feminization and testicular dysfunction.
Neuroendocrinology
Teleost fishes are particularly suitable models to study mechanisms of neuroendocrine control of reproduction because of their relatively simple neuroendocrine organization. They are unique among vertebrates in that they lack the specialized pituitary portal system present in tetrapods that delivers brain regulators to specific areas of pituitary. Instead, releasing hormones and neurotransmitter neurons directly innervate the pituitary gland in teleosts. In addition, hormone producing cells in the pituitary show a markedly distinct distribution pattern that facilitates studies on their innervation and neurotransmitter control (Fig. 3).
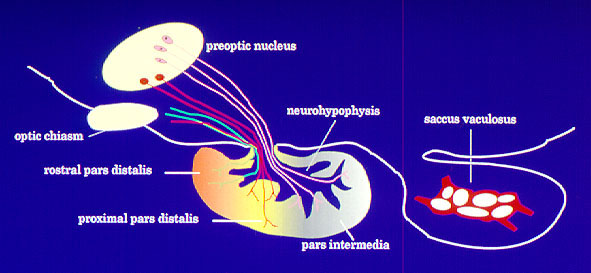
Fig. 3: Direct innervation of teleost pituitary by stimulatory and inhibitory pathways originating from preoptic anterior hypothalamic area.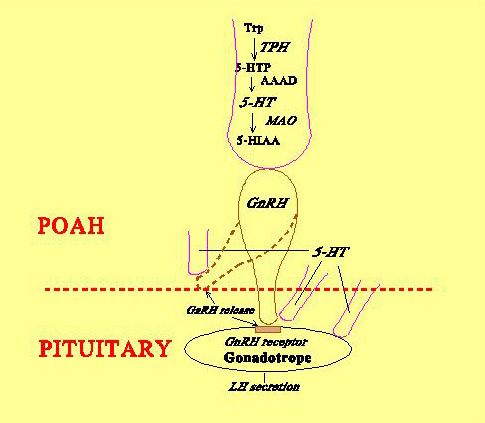 Fig. 4: Schematic diagram showing possible sites of 5-HT action on the GnRH-LH system in Atlantic croaker.Neuroendocrine control of reproduction in fishes, similar to other vertebrates, involves a complex interaction of a variety of neurotransmitters that modulate the stimulatory influence of gonadotropin-releasing hormone (GnRH) on the synthesis and release of the two gonadotropins (FSH and LH). For example, serotonin (5-hydroxytryptamine, 5-HT) exerts stimulatory influences on LH secretion by acting at multiple levels of the brain-pituitary axis (Fig. 4).
Fig. 4: Schematic diagram showing possible sites of 5-HT action on the GnRH-LH system in Atlantic croaker.Neuroendocrine control of reproduction in fishes, similar to other vertebrates, involves a complex interaction of a variety of neurotransmitters that modulate the stimulatory influence of gonadotropin-releasing hormone (GnRH) on the synthesis and release of the two gonadotropins (FSH and LH). For example, serotonin (5-hydroxytryptamine, 5-HT) exerts stimulatory influences on LH secretion by acting at multiple levels of the brain-pituitary axis (Fig. 4).
We are interested in understanding the mechanisms of neuroendocrine control of synthesis and secretion of FSH and LH in the croaker model of reproductive neuroendocrinology.Applications
Aquaculture - One of the major problems in aquaculture of most fishes is the lack of spontaneous spawning in captivity due primarily to the absence of appropriate environmental and social cues resulting in alterations of normal neuroendocrine processes. Therefore, an understanding of neuroendocrine mechanisms involved in the control of final stages of gamete maturation is essential for the development of new and improved methods of induced breeding of captive broodstocks.
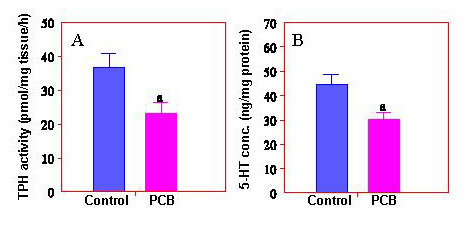 Fig. 5: PCB reduces hypothalamic TPH activity and 5-HT concentrations in Atlantic croaker.Neuroendocrine toxicology - Our research in basic neuroendocrinology has helped investigate novel mechanisms of neuroendocrine toxicity of environmental pollutants, such as PCBs, DDTs, and heavy metals that can disrupt vertebrate reproduction. These studies have established Atlantic croaker as an excellent vertebrate model to investigate mechanisms of neuroendocrine control of reproduction and perturbation of neuroendocrine pathways by environmental chemicals. A better understanding of these mechanisms will help design specific drugs to treat the problems associated with neuroendocrine impairments induced by exposure to toxic environmental chemicals. We have found for the first time in a vertebrate species, Atlantic croaker, that a polychlorinated biphenyl mixture (Aroclor 1254, PCB) impairs LH secretion by inhibiting hypothalamic tryptophan hydroxylase (TPH), the rate-limiting enzyme in 5-HT synthesis (fig 5). In addition to the evidence of neuroendocrine disruption, this finding may have broad implications for mental health in PCB exposed human populations.
Fig. 5: PCB reduces hypothalamic TPH activity and 5-HT concentrations in Atlantic croaker.Neuroendocrine toxicology - Our research in basic neuroendocrinology has helped investigate novel mechanisms of neuroendocrine toxicity of environmental pollutants, such as PCBs, DDTs, and heavy metals that can disrupt vertebrate reproduction. These studies have established Atlantic croaker as an excellent vertebrate model to investigate mechanisms of neuroendocrine control of reproduction and perturbation of neuroendocrine pathways by environmental chemicals. A better understanding of these mechanisms will help design specific drugs to treat the problems associated with neuroendocrine impairments induced by exposure to toxic environmental chemicals. We have found for the first time in a vertebrate species, Atlantic croaker, that a polychlorinated biphenyl mixture (Aroclor 1254, PCB) impairs LH secretion by inhibiting hypothalamic tryptophan hydroxylase (TPH), the rate-limiting enzyme in 5-HT synthesis (fig 5). In addition to the evidence of neuroendocrine disruption, this finding may have broad implications for mental health in PCB exposed human populations.Gonadal and Gamete Physiology
 Germinal vesicle migration stage of final oocyte maturationThe marked morphological and physiological changes associated with oocyte and sperm production which occur in the gonads during the reproductive cycle are under complex endocrine control. However, despite intensive research efforts, the hormone regulation of many aspects of gonadal and gamete functions remain poorly understood.
Germinal vesicle migration stage of final oocyte maturationThe marked morphological and physiological changes associated with oocyte and sperm production which occur in the gonads during the reproductive cycle are under complex endocrine control. However, despite intensive research efforts, the hormone regulation of many aspects of gonadal and gamete functions remain poorly understood.
Research in our laboratory has focused on the hormonal control of oocyte and sperm maturation; in particular, the roles of membrane receptors for the maturation-inducing steroid (20β-S), and gap junctions and connexin genes. We have found that fully grown oocytes need to be exposed to gonadotropin in order to become responsive to 20β-S and undergo meiotic maturation. Further, upregulation of the 20β-S receptor and gap junction formation appear to be key events in the development of oocyte responsiveness to 20β-S during the preovulatory surge in gonadotropin secretion.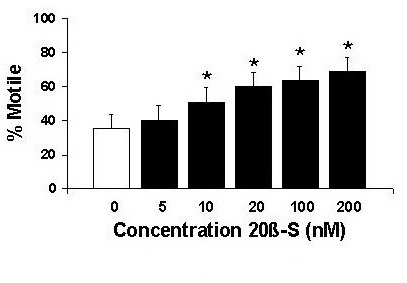 Fig. 6: Concentration-response of maturation-inducing steroid 20β-S on croaker motility.We have recently demontrated that 20β-S also exerts a direct effect on fish sperm, causing a dose-dependent increase in sperm motility after one minute exposure (fig 6). Moreover, a specific receptor for 20β-S has been identified on sperm membranes which is the likely mediator of the steroid's action.
Fig. 6: Concentration-response of maturation-inducing steroid 20β-S on croaker motility.We have recently demontrated that 20β-S also exerts a direct effect on fish sperm, causing a dose-dependent increase in sperm motility after one minute exposure (fig 6). Moreover, a specific receptor for 20β-S has been identified on sperm membranes which is the likely mediator of the steroid's action.
Another interest is the modulation of steroid hormone secretion by steroids themselves, especially those involving nongenomic mechanisms. For example, we have found that estrogens, which are produced in small amounts in the testis, down-regulate androgen production in that tissue by a nongenomic action and that several estrogenic pesticides can also act via this mechanism.

Sperm without 20β-S: note lack of motility characterized by slow movement and little turning. Sperm with 20β-S: sperm show much higher motility characterized by fast swimming in tight arcs. CURRENT PROJECTS
Characterization of membrane Progesterone Receptor
A novel membrane localized progestin receptor was first identified in spotted seatrout ovary and was shown to induce final oocyte maturation prior to spawning. An analogous protein has also been found in humans. Studies on the structure as well as the functions of the mPRs in various species and tissues continue.
Characterization of mPRs in human breast cancer
Breast cancer will affect more than one in ten women in the US. Many breast tumors are steroid dependant and while estrogen has been the main treatment target evidence is suggesting the progesterone is also important in tumor development and growth. Current work in the lab examines the roles of mPRs in human breast cancer cell lines and expression in breast tumors.
Regulation and function of a progestin receptor on fish sperm membranes (U.S. Dept. of Agriculture)
Fertilization success in fishes is greatly influenced by the quality of sperm released by the males. Sperm motility varies considerably in many important aquaculture species both between models and also within the same individual at different stages of the reproductive cycle, but their underlying causes are unknown. The purpose of this project is to obtain a better understanding of the mechanisms by which sperm activation and sperm quality are regulated by a progestin steroid hormone (20β-S) in the Atlantic croaker. Recently, we discovered that 20β-S can act directly on sperm to increase its motility and fertilization capability. Moreover, we identified a specific receptor for 20β-S on croaker sperm membranes, the first described in any fish species. We propose that this sperm progestin receptor is a critical component of the regulatory system for sperm activation. We are investigating whether progestin receptor concentrations influence hormonal activation of sperm, the intracellular changes in Ca2+ and cyclic AMP induced by binding of 20β-S to its receptor, and their effects on sperm motility and fertilization capability. We expect that fundamental aspects of this study will be broadly applicable to important aquaculture species and will be the basis for the development of effective hormonal treatments to enhance sperm quality in fish species of aquaculture importance.
Effects of Hypoxia in the Gulf of Mexico (NOAA)
 The dead zone is an area of the Gulf of Mexico off the Louisiana coast where extremely low oxygen levels (hypoxia) at the ocean floor have killed or forced out marine organisms. Increased nutrients in the Mississippi River from fertilizer runoff and sewage effluent causes a larger than normal algae bloom in the Gulf shelf waters which eventually sinks and consumes oxygen as it decomposes. As the nutrient content of the Mississippi changes seasonally the size of the dead zone waxes and wanes. In the winter it is small or undetectable and grows to the size of New Jersey in the summer.
The dead zone is an area of the Gulf of Mexico off the Louisiana coast where extremely low oxygen levels (hypoxia) at the ocean floor have killed or forced out marine organisms. Increased nutrients in the Mississippi River from fertilizer runoff and sewage effluent causes a larger than normal algae bloom in the Gulf shelf waters which eventually sinks and consumes oxygen as it decomposes. As the nutrient content of the Mississippi changes seasonally the size of the dead zone waxes and wanes. In the winter it is small or undetectable and grows to the size of New Jersey in the summer.  This area is home to many commercially and ecologically important marine species. The Atlantic croaker, a common Gulf fish and a model species for research, spawns in the late summer and autumn and a period of severe hypoxia may interrupt reproduction. This project is investigating the effects of severe hypoxia on the reproductive ability and success of Atlantic croaker.
This area is home to many commercially and ecologically important marine species. The Atlantic croaker, a common Gulf fish and a model species for research, spawns in the late summer and autumn and a period of severe hypoxia may interrupt reproduction. This project is investigating the effects of severe hypoxia on the reproductive ability and success of Atlantic croaker.
This project has both a field component to establish the natural condition and the reactions of fish to the dead zone in the wild and a lab component to determine the mechanisms behind phenomena observed in the field. This research is apart of a much larger project funded by NOAA that involves numerous scientist from several universities and various expertise in marine science.Mechanism of hypoxia down-regulation of reproductive neuroendocrine function in an estuarine teleost, Atlantic croaker (NSF)
There is growing concern over the recent dramatic increase in the incidence of seasonal hypoxia (low oxygen levels) in coastal regions worldwide due to increased nitrogen inputs through human activities. The impact of this environmental deterioration on the abundance of marine animals and valuable coastal fishery resources is unknown because basic information is lacking on the long term reproductive effects of hypoxia. Preliminary results suggest the presence of a specific mechanism in the brain of an estuarine fish, Atlantic croaker, to shut down reproduction in response to hypoxia by decreasing hormone secretion. The hypothesis that hypoxia impairs reproduction in croaker through down regulation of tryptophan hydroxylase (TPH), a critical enzyme for synthesis of serotonin, a chemical in the brain that regulates reproductive hormones, will be investigated using a variety of biochemical and molecular techniques. The results are expected to show that hypoxia impairs reproduction by a specific mechanism involving down regulation TPH activity in the brain. Moreover, it is predicted that the decrease in TPH activity is due to a decline in TPH mRNA expression which is a specific response to hypoxia and is not observed with other environmental stressors. This research is likely to have broad impacts on our understanding of reproductive adaptations of organisms to environmental changes, particularly to oxygen-limited conditions such as hypoxia, at high altitudes, and during hibernation, and for the many other physiological functions of serotonin. The demonstration that the down regulation of reproductive and endocrine functions by hypoxia is mediated through a hitherto unrecognized specific adaptation mechanism would suggest a major influence of hypoxia in the evolution and ecology of estuarine organisms such as croaker. However, the results of this study may have the greatest impact in formulating policy decisions, because identifying the precise mechanism by which hypoxia causes reproductive suppression in fish provides a compelling argument for the existence of this hypoxia effect and its ecological importance.
Role of the novel membrane progesterone receptors (mPRs) in the human vascular system (The Morris L. Lichtenstein, Jr. Medical Research Foundation)
The results of both clinical and laboratory studies indicate that progesterone and synthetic progestins can influence cardiovascular functions, but the receptor mechanisms and sites of action remain unclear. We have identified new important physiological functions of the novel membrane progesterone receptor, mPRα, in a wide variety of reproductive tissues. However, the functions of mPRα in the cardiovascular system have not been investigated. Two key components of the vascular system, endothelial cells and smooth muscle cells, are likely sites of progestin action through mPRα. Therefore, we tested the hypothesis that mPRα influences the functions of the human vascular system, using vascular cells harvested from human umbilical cords. Our results show that progesterone exerts protective functions on human vascular endothelial cells by increasing nitric oxide (NO) production. NO causes vascular smooth muscle relaxation resulting in a decrease in blood pressure. Our results show this protective function of progesterone is mediated through membrane progesterone receptor alpha.
-
PUBLICATIONS IN PRESS
None at this time
PUBLICATIONS
278 Pang Y., Thomas, P. 2018. Progesterone induces relaxation of vascular smooth muscle cells through mPRα (PAQR7). Molecular and Cellular Endocrinology. 474: 20-34.
277 Ondricek, K., Thomas, P. 2018. Effects of hypoxia exposure on apoptosis and expression of membrane steroid receptors, ZIP9, mPRα, and GPER in Atlantic croaker ovaries. Comparative Biochemistry and Physiology, A.224:84-92.
276 Sinreih, M., Knific, T., Thomas, P., Frković Grazio, S., Lanišnik Rižner, T. 2018. Membrane progesterone receptors β and γ have potential as prognostic biomarkers of endometrial cancer. Journal of Steroid Biochemistry and Molecular Biology 178:303-311.
275 Rahman, Md.S.,Thomas, P. 2018. Interactive effects of hypoxia and PCB co-exposure on expression of CYP1A and its potential regulators in Atlantic croaker liver. Environmental Toxicology, 1-11. DOI: 10.1002/tox.22527
274 Aizen, J., Pang, Y., Harris, C., Converse, A., Zhu, Y., Aguirre, M., Thomas, P. 2018. Roles of progesterone receptor membrane component 1 and membrane progestin receptor alpha in regulation of zebrafish oocyte maturation. General and Comparative Endocrinology 263: 51-61.
273 Rose, K.A., Creekmore, S.,Thomas, P., Craig, J.K., Rahman, Md. S, Miller-Neilan, R. 2018. Modeling the population effects of hypoxia on Atlantic croaker (Micropogonias undulatus) in the northwestern Gulf of Mexico: Part 1 – Model description and idealized hypoxia. Estuaries and Coasts 41:233-254.
272 Rose, K.A., Creekmore, S., Justic, D., Thomas, P., Craig, J.K., Miller-Neilan, R., Wang, L., Rahman, Md. S., Kidwell, D. 2018. Modeling the population effects of hypoxia on Atlantic croaker (Micropogonias undulatus) in the northwestern Gulf of Mexico: Part 2 – Realistic hypoxia and eutrophication- Estuaries and Coasts 41: 255-279.
271 Barton, M., Filardo, E.D., Lolait, S.J., Thomas, P., Maggiolini, M., Prossnitz, E.R. 2018. Twenty years of G protein-coupled estrogen receptor: historical and personal perspectives. Journal Steroid Biochemistry and Molecular Biology 176:4-15.
270 Thomas, P., Converse, A., Berg, H.A. 2018. ZIP9, a novel membrane androgen receptor and zinc transporter protein. General and Comparative Endocrinology 257: 130-136.
269 Rahman, Md. S., Thomas, P. 2017. Molecular and biochemical responses to hypoxia exposure in Atlantic croaker collected from hypoxic regions in the northern Gulf of Mexico. PloS ONE 12 (9). e0 0184341.
268 Converse, A., Zhang, C., Thomas, P. 2017. Membrane androgen receptor ZIP9 induces croaker ovarian cell apoptosis via stimulatory G protein alpha subunit and MAP kinase signaling. Endocrinology 158:3015-3029.
267 Thomas, P., 2017. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes. Journal of Steroid Biochemistry and Molecular Biology. 167:153-161.
266 Thomas, P., Pang, Y., Dong, J. 2017. Membrane androgen receptor characteristics of human ZIP9 (SLC39A) zinc transporter in prostate cancer cells: androgen-specific activation and involvement of an inhibitory G protein in zinc and MAP kinase signaling. Molecular and Cellular Endocrinology 447:23-34.
265 Pang, Y., and Thomas, P. 2017. Additive effects of low concentrations of estradiol-17β and progesterone on nitric oxide production by human vascular endothelial cells through shared signaling pathways. Journal of Steroid Biochemistry and Molecular Biology 165:258-267.
264 Shi, B., X. Liu, P. Thomas, Y. Pang, Y. Xu, X. Li, X. Li. 2016. Identification and characterization of a progestin and adipoQ receptor (PAQR) structurally related to Paqr7 in the ovary of Cynoglossus semilaevis and its potential role in regulating oocyte maturation. General and Comparative Endocrinology 237:109-120.
263 Mankarious, A., Dave, F., Pados, G., Tsolakidis, D., Y. Gidron, Pang, Y., Thomas, P., Hall, M., Karteris, E. 2016. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. International Journal of Oncology 48:1805-1814.
262 Fitzgerald, A.C., C. Peyton, J. Dong, and P. Thomas. 2015. Bisphenol A and related alkylphenols exert nongenomic estrogenic actions through a G protein-coupled estrogen receptor 1 (Gper)/epidermal growth factor receptor (Egfr) pathway to inhibit meiotic maturation of zebrafish oocytes. Biology of Reproduction. 93 960 147: 1-9.
261 Thomas, P., Md. S. Rahman, M., Picha, and W. Tan. 2015. Impaired gamete production and viability in Atlantic croaker collected throughout the 20,000 km2 hypoxic region in the northern Gulf of Mexico. Marine Pollution Bulletin. 101:182-192.
260 Tan, W., and P. Thomas. 2015. Involvement of epidermal growth factor receptors and mitogen-activated protein kinase in progestin induction of sperm hypermotility in Atlantic croaker through membrane progestin receptor-alpha. Molecular and Cellular Endocrinology (DOI: 10.1016/j.mce.2015.06.023).
259 Rahman, M.S. and P. Thomas. 2015. Molecular characterization and hypoxia-induced upregulation of neuronal nitric oxide >synthase in Atlantic croaker: reversal by antioxidant and estrogen treatments. Comparative Biochemistry and Physiology: Part A. 185:91-106.
258 Pang, Y., J. Dong, and P. Thomas. 2015. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor alpha (mPRα). American Journal of Physiology; Endocrinology and Metabolism 308: E899-E911.
257 Aizen, Y. and P. Thomas. 2015. Involvement of progesterone membrane component 1 (PGRMC1) in estrogen maintenance of meiotic arrest in zebrafish oocytes through regulation of epidermal growth factor receptor expression on oocyte membranes. Journal of Endocrinology 225:59-68.
256 Chourasia, T., Y. Pang, P. Thomas. 2015. The catecholestrogen, 2-hydroxyestradiol-17 beta, acts as a G protein-coupled estrogen receptor 1 (GPER) antagonist to promote the resumption of meiosis in zebrafish oocytes. Biology of Reproduction 92:69.1-13.
255 Rahman, Md. S and P.Thomas. 2014. Restoration of tryptophan hydroxylase functions and serotonin content in Atlantic croaker hypothalamus by antioxidant treatment during hypoxic stress. Frontiers in Neuroscience 8: article 150:1-12.
254 Berg, A.H., C.D. Rice, Md. S. Rahman, J. Dong, and P. Thomas. 2014. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female Atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology 155:4237-4249.
253 Thomas, P., J. Dong, A.H. Berg, and Y.F. Pang. 2014. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155:4250-4265.
252 Foster H.A., J. Davies, R.C. Pink, S. Turckigdem, A. Goumenou, D.R. Cater, N.J. Saunder, P. Thomas, and E. Karteris. 2014. The human myometrium differentially expresses mTOR signalling components before and during pregnancy: evidence for regulation by progesterone. Journal Steroid Biochemistry and Molecular Biology 139:166-172.
251 Mohan, J., Md. S. Rahman, P. Thomas, and W. Walther. 2014. Influence of constant and periodic hypoxic stress in controlled laboratory experiments on Atlantic croaker otolith chemistry. Aquatic Biology 20:1-11.
250 Cheng, S-B., J. Dong, Y. Pang, M. Hixson, P. Thomas, and E.J. Filardo. 2014. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Molecular and Cellular Endocrinology 382:950-959.
249 Thomas, P., Y. Pang, and J. Dong. 2014. Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 155:1107-1119.
248 Tan, W., J. Aizen, and P. Thomas. 2014. Membrane progestin receptor alpha mediates progestin-induced sperm hypermotility and increased fertilization success in southern flounder ( General and Comparative Endocrinology 200:18-26.
247 Tan, W. and P. Thomas. 2014. Activation of the Pi3k/Akt pathway and modulation of phosphodiesterase activity via membrane progestin receptor-alpha (mPRalpha) regulate progestin-initiated sperm hypermotility in Atlantic croaker. Biology of Reproduction 90 (5):1-11.
246 Wang, J., R. Yamamoto Y. Yamamoto, T. Tokumoto, J. Dong, P. Thomas, H. Hirai, and H. Kawagishi. 2013. Hydroxylation of bisphenol A by hyper lignin-degrading fungus Phanerochaete sordidia YK-624 under non-ligninolytic condition. Chemosphere 93:1419-1423.
245 Rahman, M.S. and P. Thomas. 2013. Interactive effects of hypoxia with estradiol-17β on tryptophan hydroxylase activity and serotonin levels in the Atlantic croaker hypothalamus. General and Comparative Endocrinology 192: 71-76.
244 Johnstone, W.M. III, K.A. Mills, R.A. Alyea, P. Thomas, and R.J. Borski. 2013. Characterization of membrane receptor binding activity for cortisol in the liver and kidney of the euryhaline teleost, Mozambique Tilapia (Oreochromis mossambicus). General and Comparative Endocrinology 192:107-114.
243 Thomas P. and Y. Pang. 2013. Protective functions of progesterone in the cardiovascular system: Potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids 78:583-588.
242 Pang Y., J. Dong, and P. Thomas. 2013. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptorsδ and ε (mPRδ and mPRε) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology 154:283-295.
241 Zuloaga, D.G., S. Yahn, Y. Pang, A. Quihuis, P. Thomas, R.J. Handa, and S.K. Mani. 2012. Distribution and estrogen regulation of membrane progesterone receptor beta in the female rat brain. Endocrinology 153:4432-4443.
240 Ropero, A.B., Y. Pang, P. Alonso-Magdalena, P. Thomas, and A Nadal. 2012. Role of ER and GPR30 in the endocrine pancreas: a matter of estrogen dose. Steroids 77:951-958.
239 Ndiaye, K., D.H. Poole, S. Walusimbi, M.J. Cannon, K. Toyokawa, S.W. Maalouf, J. Dong, P. Thomas, and P.L. Pate. 2012. Progesterone effects on bovine lymphocytes are potentially mediated by membrane progesterone receptors (mPRs). Journal Reproductive Immunology 95:15-26.
238 Kodama, K., Md. S. Rahman, T. Horiguchi, and P. Thomas. 2012. Effects of environmental hypoxia exposure on transcript levels of two hypoxia-inducible factor α genes (HIF-1α and HIF-2α) in a marine teleost dragonet Callionymus valenciennei from Tokyo Bay. Marine Pollution Bulletin 64:1339-1347.
237 Thomas P. and Y. Pang. 2012. Membrane progesterone receptors (mPRs): evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology 96:162-171.
236 Rahman, M.S. and P. Thomas. 2012. Effects of hypoxia exposure on hepatic cytochrome P4501A (CYP1A) expression in Atlantic croaker: mechanisms of CYP1A down-regulation. PLoS ONE 7(7):e40285.
235 Zachariades E., D. Mparmpakas, P. Thomas, M. Rand-Weaver, and E. Karteris. 2012. Crucial cross-talk of interleukin-1 beta and progesterone in human choriocarcinoma. International Journal of Oncology 40:1358-1364.
234 Filardo, E.J. and P. Thomas. 2012. Minireview: G-protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and physiological role in female reproductive cancer and renal physiology. Endocrinology 153:2953-2962.
233 Picha, M.E., B. Shi, and P. Thomas. 2012. Dual role of IGF-II in oocyte maturation in southern flounder Paralichthys lethostigma: Up-regulation of mPRα and resumption of meiosis. General and Comparative Endocrinology 177:220-230.
232 Zachariades, E., D. Mparmpakas, Y. Pang, M. Rand-Weaver, P. Thomas, E. Karteris. 2012. Changes in placental membrane progesterone receptors in term and preterm labour. Placenta 33:367-37.
231 Tokumoto T., M. Tokumoto, T. Oshima, K. Shimizuguchi, T. Fukuda, E. Sugita, M. Suzuki, Y-t Sakae, Y-I Akiyama, R. Nakayama, S.R. Roy, Md. S. Rahman, Y. Pang, J. Dong, and P. Thomas. 2012. Characterization of multiple membrane progestin receptor (mPR) sub-types from the goldfish ovary and their roles in the induction of oocyte maturation. General and Comparative Endocrinology 177:168-176.
230 Dressing G.E., R. Alyea, Y. Pang, and P. Thomas. 2012. Membrane progesterone receptors (mPRs) mediate antimorbidity in breast cancer cells and are expressed in human breast tumors. Hormones and Cancer 3:101-112.
229 Kodama K., M.S. Rahman, T. Horiguchi, and P. Thomas. 2012. Assessment of hypoxia-inducible factor-1α mRNA expression in mantis shrimp as a biomarker of environmental hypoxia exposure. Marine Biology Letters 8:278-281.
228 Thomas, P. 2012. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. General and Comparative Endocrinology 175:367-383.
227 Thomas, P. and M.S. Rahman. 2012. Extensive reproductive disruption, ovarian masculinization, and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proceedings Royal Society B 279, 28-38.
226 Rahman, M.S. and P. Thomas. 2011. Characterization of three IGFBP mRNAs in Atlantic croaker and their regulation during hypoxic stress: potential mechanisms of their upregulation by hypoxia. American Journal Physiology: Endocrinology and Metabolism 301:E637-E648.
225 Rahman, M.S., I.A. Khan, P. Thomas. 2011. Tryptophan hydroxylase: a target of neuroendocrine disruption. Journal of Toxicology and Environmental Health, Part B, 14:5-7, 473-494.
224 Pang, Y.F. and P. Thomas. 2011. Progesterone signals through membrane progesterone receptors (mPRs) in MDA-MB-468 and mPR-transfected MDA-MB-231 breast cancer cells which lack full-length and C-terminal truncated isoforms of the nuclear progesterone receptor. Steroids 76:921-928.
223 Peyton, C. and P. Thomas. 2011. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio). Biology of Reproduction 85:42-50.
222 Zachariades, E., H. Foster, A. Gouenou, P. Thomas, M. Rand-Weaver, and E. Karteris. 2011. Expression of membrane and nuclear progesterone receptors in two human placental choriocarcinoma cell lines (JEG-3 and BeWo): effects of syncytialization. International Journal of Molecular Medicine 27:767-774.
221 Tubbs, C., W. Tan, B. Shi, and P. Thomas. 2011. 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) stimulation of sperm hypermotility and identification of 20β-S binding and membrane progestin receptor alpha on southern flounder sperm (Paralichthys lethostigma). General and Comparative Endocrinology 170: 629-639.
220 Patel, V.H., J. Chen, M. Ramanjaneya, E. Karteris, E. Zachariades, P. Thomas, M. Been, and H.S. Randeva. 2010. G-protein-coupled estrogen receptor 1, GPER, Expression in rat and human heart: protective role during ischaemic stress. International Journal of Molecular Medicine 26:193-199.
219 Thomas P. and Md. S. Rahman. 2010. Region-wide impairment of Atlantic croaker testicular development and sperm production in the northern Gulf of Mexico hypoxic dead zone. Marine Environmental Research 69:S59-62.
218 Charles, N.A., P. Thomas, and C.A. Lange. 2010. Signaling events mediated by membrane progesterone receptors (mPRs) in ovarian cancer cells. Hormones and Cancer 1:167-176.
217 Dressing, G., Y. Pang, J. Dong, and P. Thomas. 2010. Progestin signaling through mPRα in Atlantic croaker granulosa/theca cell co-cultures and its involvement in progestin inhibition of apoptosis. Endocrinology 151:5914-5928.
216 Pang, Y.F. and P. Thomas. 2010. Role of G protein estrogen receptor 1, GPER, in inhibition of spontaneous maturation of zebrafish oocytes by endogenous estrogens. Developmental Biology 342:194-206.
215 Tubbs, C., M. Pace, and P. Thomas. 2010. Expression and gonadotropin regulation of membrane progestin receptor alpha in Atlantic croaker ( General and Comparative Endocrinology 165:144-145.
214 Thomas P., R. Alyea, Y. Pang, C. Peyton, J. Dong, and H. Berg. 2010. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1, GPER, in mammals and fish. Steroids 75:595-602.
213 Rossi D.V., Y. Dai, P. Thomas, G.A. Carrasco, L.L. Doncarlos, and N.A. Muma, 2010. Estradiol-induced desensitization of 5-HT (1A) receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinology 35:1023-1033.
212 Foster H., A. Reynolds, G. Stenbeck, J. Dong, P. Thomas, and E. Karteris. 2010. Internalization of membrane progesterone receptor alpha (mPRα) after treatment with progesterone: potential involvement of a clathrin-dependent pathway. Molecular Medicine Reports 3:27-35.
211 Labombarda, F., D. Meffre, B. Delespierre, S. Krivokapic-Blondiaux, A. Chastre, P. Thomas, Y. Pang, J.P. Lydon, S.L. Gonzalez, A.F. De Nicola, M. Schumacher, and R. Guennoun. 2010. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 166:94-106.
210 Kelder, J., R. Azevedo, Y. Pang, J. de Vlieg, J. Dong, and P. Thomas. 2010. Comparison between steroid binding to the human progestin membrane receptor α subtype and to the human progestin nuclear receptor: correlations with physicochemical properties assessed by comparative molecular field analyses. Steroids 75: 314-322.
209 Hanna, R.N., S.C.J. Daly, Y. Pang, I. Anglade, O. Kah, P. Thomas, and Y. Zhu. 2010. Characterization and expression of nuclear progesterone receptor in zebrafish gonads and brain. Biology of Reproduction 82:112-122.
208 Rose, K.A, S.E. Sable, A.T. Adamack, C.A. Murphy, S E. Kolesar, J.K. Craig, D. L. Breitburg, P. Thomas, M.H. Brouwer, and C.F. Cerco. 2009. Does hypoxia have population-level effects on coastal fish? Musing from the virtual world. Journal Experimental Marine Biology and Ecology 381:S188-S203.
207 Thomas, P. and Md. S. Rahman. 2009. Biomarkers of hypoxia exposure and reproductive function in Atlantic croaker: a review with some preliminary findings from the Northern Gulf of Mexico hypoxic zone. Journal Experimental Marine Biology and Ecology 381:S38-S50.
206 Nutu, M., B. Weijdegard, P. Thomas, A. Thurin-Kjellberg, H. Billig, and D.G.J. Larsson. 2009. Distribution and hormonal regulation of membrane progesterone receptors β and γ in ciliated epithelial cells of mouse and human fallopian tubes. Reproductive Biology and Endocrinology 7:89.
205 Sleiter, N., Y. Pang, C. Park, T.H. Horton, J. Dong, P. Thomas, and J.E. Levine. 2009. Progesterone receptor A (PRA) and PRB –independent effects of progesterone on GnRH release. Endocrinology 150:3833-44.
204 Thomas, P. and Md. S. Rahman. 2009. Chronic hypoxia impairs gamete maturation in Atlantic croaker induced by progestins through nongenomic mechanisms resulting in reduced reproductive success. Environmental Science and Technology 43:4175-80.
203 Thomas, P., C. Tubbs and V.F. Garry. 2009. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): identification of mPRα on human sperm and its association with sperm motility. Steroids 74:614-621.
202 Murphy, C.A., K.A. Rose, Md. S. Rahman, and P. Thomas. 2009. Hypoxia as an endocrine disruptor: testing and applying a fish vitellogenesis model to evaluate lab and field biomarkers of hypoxia. Environmental Toxicology and Chemistry 28:1288-1303.
201 Rahman, Md. S. and P. Thomas. 2009. Molecular cloning, characterization and expression of two tryptophan hydroxylase (TPH-1 and TPH-2) genes in the hypothalamus of Atlantic croaker: down-regulation after chronic exposure to hypoxia. Neuroscience 158:751-765.
200 Tubbs, C. and P. Thomas. 2009. Progestin signaling through an olfactory G protein and membrane progestin receptor alpha in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology 150:473-484.
199 Pang, Y.F. and P. Thomas. 2009. Involvement of estradiol-17β and its membrane receptor, G protein coupled receptor 30 (GPR30) in regulation of oocyte maturation in zebrafish, Danio rerio. General and Comparative Endocrinology 161:58-61.
198 Zhu, Y., R.N Hanna, M.J.M. Schaaf, H.P. Spaink, and P. Thomas. 2008. Candidates for membrane progestin receptors in vertebrates-past approaches and future challenges. Comparative Biochemistry and Physiology C, Toxicology and Pharmacology 148:381-389.
197 Tokumoto, T., M. Tokumoto, and P. Thomas. 2008. Induction of fish oocyte maturation by DES through membrane progestin receptor. Cybium 32(2):153-155.
196 Thomas, P. 2008. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component one (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in Neuroendocrinology 29:292-312.
195 Thomas, P. and J. Sweatman. 2008. Interference by atrazine and bisphenol A with progestin binding to the ovarian progestin membrane receptor and induction of oocyte maturation in Atlantic croaker. Marine Environmental Research 66:1-2.
194 Tubbs, C. and P. Thomas. 2008. Functional characteristics of membrane progestin receptor alpha (mPRα) subtypes: A review with new data showing mPRα expression in seatrout sperm and its association with sperm motility. Steroids 73:935-941.
193 Dosiou, C., A.E. Hamilton, Y. Pang, M.T. Overgaard, S. Tulac, J. Dong, P. Thomas, and L.C. Guidice. 2008. Expression of membrane progesterone receptors (mPRs) on human T lymphocytes and Jurkat cells and activation of G proteins by progesterone. J. Endocrinol. 196:67-71.
192 Pang, Y.F., J. Dong and P. Thomas. 2008. Estrogen signaling characteristics of Atlantic croaker GPR30 and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology 149:3410-3426.
191 Thomas, P., M.S. Rahman, I.A. Khan, and J. Kummer. 2007. Widespread endocrine disruption and reproductive impairment in an estuarine fish population exposed to seasonal hypoxia. Proceedings Royal Society B 274:2693-2701.
190 Filardo, E., J. Quinn, Y. Pang, C. Graeber, S. Shaw, J. Dong, and P. Thomas. 2007. Sequestration and redistribution of the novel estrogen receptor, GPR30, from the plasma membrane following estrogen stimulation. Endocrinology 148:3236-3245.
189 Rahman, M.S. and P. Thomas. 2007. Molecular cloning, characterization and expression of two hypoxia-inducible transcription factors (HIF-1α and HIF-2α) in a hypoxia-tolerant marine teleost, Atlantic croaker ( Gene 396:273-282.
188 Nutu, M., B. Weijdegård, P. Thomas, C. Bergh, A. Thurin-Kjellberg, Y.F. Pang, H. Billig, and D.G. Larsson. 2007. Membrane progesterone receptor gamma: tissue distribution and expression in ciliated cells in the fallopian tube. Molecular Reproduction and Development 74:843-850.
187 Tokumoto, T., M. Tokumoto and P. Thomas. 2007. Interactions of diethylstilbestrol (DES) and DES analogues with membrane progestin receptor α (mPRα) and the correlation with their nongenomic progestin activities. Endocrinology 148:3459-3467.
186 Ben-Yehoshua, L.F., A.L. Lewellyn, P. Thomas, and J.L. Maller. 2007. The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Molecular Endocrinology 21:664-673.
185 Dressing, G.E. and P. Thomas. 2007. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids 72:111-116.
184 Thomas, P., Y. Pang, J. Dong, J. Groenen, J. Kelder, H. van de Vlieg, Y. Zhu, and C. Tubbs. 2007. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology 148:705-718.
183 Thomas, P. and J. Dong. 2006. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. Journal of Steroid Biochemistry and Molecular Biology 102:107-109.
182 LeRoy, K.D., P. Thomas and I.A. Khan. 2006. Thyroid hormone status of Atlantic croaker exposed to Aroclor 1254 and selected PCB congeners. Comparative Biochemistry and Physiology C 144:263-271.
181 Kucherka, W.D., P. Thomas and I.A. Khan. 2006. Sex differences in circulating steroid hormone levels in the red drum, Sciaenops ocellatus. Aquaculture Research 37:1464-1472.
180 Hanna, R., Y. Pang, P. Thomas, and Y. Zhu. 2006. Cell surface expression, progestin binding and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. Journal of Endocrinology 190:247-260.
179 Benninghoff, A. and P. Thomas. 2006. Gonadotropin regulation of testosterone production by primary cultured theca and granulosa cells of Atlantic croaker: I. Novel role of CaMKs and interactions between calcium- and adenylyl cyclase-dependent pathways. General and Comparative Endocrinology 147:276-287.
178 Benninghoff, A. and P. Thomas. 2006. Gonadotropin regulation of testosterone production by primary cultured theca and granulosa cells of Atlantic croaker: II. Involvement of a mitogen-activated protein kinase pathway. General and Comparative Endocrinology 147:288-297.
177 Thomas, P., Md. S. Rahman, J.A. Kummer, and S. Lawson. 2006. Reproductive endocrine dysfunction in Atlantic croaker exposed to hypoxia. Marine Environmental Research 62:249-252.
176 Khan I.A. and P. Thomas. 2006. PCB-congener specific disruption of reproductive neuroendocrine function in Atlantic croaker. Marine Environmental Research 62:25-28.
175 Karteris, E., S. Zervou, Y.F. Pang, J. Dong, E.W. Hillhouse, H.S. Randeva, and P. Thomas. 2006. Progesterone signaling in human myometrium through two novel membrane G protein couple receptors: potential role in functional progesterone withdrawal at term. Molecular Endocrinology 20(7):1519-1534.
174 Larsson, D.G.J., M. Adolfsson-Erici and P. Thomas. 2006. Characterization of putative ligands for a fish gonadal androgen receptor in a pulp mill effluent. Environmental Toxicology and Chemistry 25(2):419-427.
173 Thomas, P., G. Dressing, Y. Pang, H. Berg, C. Tubbs, A. Benninghoff, and K. Doughty. 2006. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids 71:310-316.
172 Tokumoto, M., Y. Nagahama, P. Thomas, and T. Tokumoto. 2006. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. General and Comparative Endocrinology 145: 101-108.
171 Pace, M.C. and P. Thomas. 2005. Steroid-induced oocyte maturation in Atlantic croaker is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biology of Reproduction 73:988-996.
170 Berg, A.H., P. Thomas and P.-E. Olsson. 2005. Characterization of a membrane progestin receptor in arctic charr ovaries. Reproductive Biology and Endocrinology 3:64.
169 Benninghoff, A.D. and P. Thomas. 2005. Involvement of calcium and calmodulin in the regulation of ovarian steroidogenesis in Atlantic croaker (Micropogonias undulatus) and modulation by Aroclor 1254. General and Comparative Endocrinology 144:211-224.
168 Filardo, E.J. and P. Thomas. 2005. GPR30: a seven-transmembrane-spanning estrogen receptor (7-mER) that triggers EGF release. TRENDS in Endocrinology and Metabolism 16:362-367.
167 Pace, M.C. and P. Thomas. 2005. Activation of a pertussis toxin sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Developmental Biology 285:70-79.
166 Olsson, P.-E., A.H. Berg, J. von Hofsten, B. Grahn, A. Hellqvist, A. Larsson, J. Karlsson, C. Modig, B. Borg, and P. Thomas. 2005. Molecular cloning and characterization of a nuclear androgen receptor activated by 11-ketotestosterone. Reproductive Biology and Endocrinology 3:37.
165 Kazeto, Y., R. Goto-Kazeto, P. Thomas, and J.M. Trant. 2005. Molecular characterization of three forms of putative membrane-bound progestin receptors and their tissue-distribution in channel catfish, Ictalurus punctatus. J. Molecular Endocrinology 34:781-791.
164 Thomas, P., C. Tubbs, C. Detweiler, S. Das, L. Ford, and D. Breckenridge-Miller. 2005. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids 70:427-433.
163 Mohamed, J.S., P. Thomas and I.A. Khan. 2005. Isolation, cloning and expression of three prepro-GnRH mRNAs in Atlantic croaker brain and pituitary. Journal Comparative Neurology 488:384-395.
162 Hawkins, M.B., J. Godwin, D. Crews, and P. Thomas. 2005. The distribution of the duplicate oestrogen receptors ER beta-a and ER beta-b in the forebrain of the Atlantic croaker (Micropogonias undulatus) indicates subfunctionalization after gene duplication. Proceedings Royal Society, Part B 272:633-641.
161 Yueh, W.S., P. Thomas and C.F. Chang. 2005. Identification and secretion of 17, 20β, 21-trihydroxy-4-pregnen-3-one during oocyte maturation in protogynous black porgy, Acanthopagrus schlegeli. General and Comparative Endocrinology 140:184-191.
160 Thomas, P., Y. Pang, E.J. Filardo, and J. Dong. 2005. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology 146:624-632.
159 Murphy, C.A., K.A. Rose and P. Thomas. 2005. Modeling vitellogenesis in female fish exposed to environmental stressors: predicting the effects of endocrine disturbance due to exposure to a PCB mixture and cadmium. Reproductive Toxicology 19(3):395-409.
158 Patiño, R., D. Bolamba, P. Thomas, and N. Kumakura. 2005. Effects of external pH on hormonally regulated ovarian follicle maturation and ovulation in croaker. General and Comparative Endocrinology 141:126-134.
157 Thomas, P. and K. Doughty. 2004. Disruption of rapid, nongenomic steroid actions by environmental chemicals: Interference with progestin stimulation of sperm motility in Atlantic croaker. Environmental Science and Technology 38:6328-6332.
156 Sorensen, P.W., C.A. Murphy, K. Loomis, P. Maniak, and P. Thomas. 2004. Evidence that 4-pregnen-17,20beta,21-triol-3-one functions as a maturation-inducing hormone and pheromonal precursor in the percid fish, Gymnocephalus cernuus. Gen. Comp. Endocrinol. 139(1):1-11.
155 Khan, I.A. and P. Thomas. 2004. Aroclor 1254 inhibits tryptophan hydroxylase activity in rat brain. Archives of Toxicology 78:316-320.
154 Thomas, P. 2004. Discovery of a new family of membrane progesterone receptors in vertebrates and identification of the beta subtype in mouse brain and testis. J. Medicinal Chemistry 13:202-209.
153 Hawkins, M.B. and P. Thomas. 2004. The unusual binding properties of the third distinct estrogen receptor subtype ERßa are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinology 145:2968-2977.
152 Braun, A. and P. Thomas. 2004. Biochemical characterization of a membrane androgen receptor in the ovary of the Atlantic croaker. Biology of Reproduction 71:146-155.
151 Thomas, P., Y. Pang, Y. Zhu, C. Detweiler, and K. Doughty. 2004. Multiple rapid progestin actions and progestin membrane subtypes in fish. Steroids 69:567-574.
150 Thomas, P. 2004. Nongenomic steroid actions initiated at the cell surface: lessons from studies in fish. Fish. Biochem. Physiol. 28:3-12.
149 Patiño, R., P. Thomas and G. Yoshizaki. 2004. Ovarian follicle maturation and ovulation: an integrated perspective. Fish. Biochem.Physiol. 28:305-308.
148 Khan, I.A. and P. Thomas. 2004. Vitamin E co-treatment reduces Aroclor 1254-induced impairment of reproductive neuroendocrine function in Atlantic croaker. Marine Environmental Research 58:333-336.
147 Crews, D., O. Putz, P. Thomas, T. Hayes, and K. Howdeshell. 2003. Wildlife as models for the study of how mixtures, low doses, and the embryonic environment modulate the action of endocrine disrupting chemicals. Pure and Applied Chemistry 75(11-12):2305-2320.
146 Braun, A. and P. Thomas. 2003. Androgens inhibit estradiol-17ß synthesis in Atlantic croaker (Micropogonias undulatus) ovaries by a nongenomic mechanism initiated at the cell surface. Biology of Reproduction 69:1642-1650.
145 Orlando, E.F., G.A. Binczik, P. Thomas and L.J. Gillette, Jr. 2003. Reproductive seasonality of the male Florida gar, Lipisoteusplatyrhincus. General and Comparative Endocrinology 131:365-371.
144 Zhu, Y., C.D. Rice, Y.F. Pang, M. Pace, and P. Thomas. 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. 100(5):2231-2236.
143 Zhu, Y., J. Bond, and P. Thomas. 2003. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. 100:2237-2242.
142 Rose, K.A., C.A. Murphy, S.L. Diamond, L.A. Fuiman, and P. Thomas. 2003. Using nested models and laboratory data for predicting population effects of contaminants on fish: A step toward a bottom-up approach for establishing causality in field studies. Human and Ecological Risk Assessment 9:231-257.
141 Patiño, R., G. Yoshizaki, D. Bolamba, and P. Thomas. 2003. Role of Arachidonic acid and protein kinase C during maturation-inducing hormone-dependent meiotic resumption and ovulation in ovarian follicles of Atlantic croaker. Biology of Reproduction 68:516-523.
140 Larsson, D.G.J., T.S. Sperry and P. Thomas. 2002. Regulation of androgen receptors in Atlantic croaker brains by testosterone and estradiol. General and Comparative Endocrinology 128:224-230.
139 Mathews, S, I.A. Khan and P. Thomas. 2002. Effects of maturation-inducing steroid on LH secretion and the GnRH system at different stages of the reproductive cycle in Atlantic croaker. General and Comparative Endocrinology 126:287-297.
138 Thomas, P., Y. Zhu and M. Pace. 2002. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: A review with some new findings. Steroids 67:511-517.
137 Yoshizaki, G., R. Patiño, P. Thomas, D. Bolamba, and X. Chang. 2001. Effects of maturation-inducing hormone on heterologous gap junction coupling in ovarian follicles of Atlantic croaker. General and Comparative Endocrinology 124:359-366.
136 Patiño, R., G. Yoshizaki, P. Thomas, and H. Kagawa. 2001. Gonadotropic control of ovarian follicle maturation: The two-stage concept and its mechanisms. Comparative Biochemistry and Physiology B 129:427-439.
135 Khan, I.A., S. Mathews, K. Okuzawa, H. Kagawa, and P. Thomas. 2001. Alterations in the GnRH LH system in relation to gonadal stage and Aroclor 1254 exposure in Atlantic croaker. Comparative Biochemistry and Physiology 129:251-260.
134 Wainwright, S.E., M.A. Mora, J.L. Sericano, and P. Thomas. 2001. Chlorinated hydrocarbons and biomarkers of exposure in wading birds and fish of the Lower Rio Grande Valley, Texas. Archives Toxicol 255:201-214.
133 Thomas, P., J. Pinter and S. Das. 2001. Upregulation of the maturation-inducing steroid membrane receptor in spotted seatrout ovaries by gonadotropin during oocyte maturation and its physiological significance. Biology of Reproduction 64:21-29.
132 Khan, I.A. and P. Thomas. 2001. Disruption of neuroendocrine control of luteinizing hormone secretion by Aroclor 1254 involves inhibition of hypothalamic tryptophan hydroxylase activity. Biology of Reproduction 64:955-964.
131 Sperry, T.S. and P. Thomas. 2000. Androgen binding profiles of two distinct nuclear androgen receptors in Atlantic croaker (Micropogonias undulatus). Journal of Steroid Biochemistry and Molecular Biology 72:93-103.
130 Carlson, B.A., C.D. Hopkins and P. Thomas. 2000. Androgen correlates of socially induced changes in the electric organ discharge waveform of a momyrid fish. Hormones and Behavior 38:177-186.
129 Khan, I.A. and P. Thomas. 2000. Lead and Aroclor 1254 disrupt reproductive neuroendocrine function in Atlantic croaker. Marine Environmental Research 50:19 123.
128 Hawkins, M.B., J.W. Thornton, D. Crews, J.K. Skipper, A. Dotte, and P. Thomas 2000. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proceedings National Academy of Sciences, USA 97(2):10751-10756.
127 Chang, X., R. Patiño, G. Yoshizaki, P. Thomas, and V.H. Lee. 2000. Hormonal regulation and cellular distribution of Cx 32.2 and Cx 32.7 in the ovary of Atlantic croaker (Micropogonias undulatus). General and Comparative Endocrinology 120:146-156.
126 Thomas, P. 2000. Chemical interference with the genomic and nongenomic actions of steroids in fishes: role of receptor binding. Marine Environmental Research 50:127-134.
125 Cheek, A.O., P. Thomas and C.V. Sullivan. 2000. Sex steroids relative to alternative mating behaviors in the simultaneous hermaphrodite Serranus subligarius (Perciformes: Serranidae). Hormones and Behavior 37:198-211.
124 Loomis, A.K. and P. Thomas. 2000. Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biology of Reproduction 62:995-1004.
123 Yoshizaki, G., W. Jin, R. Patiño, and P. Thomas. 2000. Structural organization of the Atlantic croaker Cx32.2 gene and its 5' flanking region. Marine Biotechnology 2:154-160.
122 Banks, S.D., P. Thomas and K.N. Baer. 1999. Seasonal variations in hepatic and ovarian zinc concentrations during the annual reproductive cycle in female channel catfish (Ictalurus punctatus). Comparative Biochemistry and Physiology Part C 124:65-72.
121 Sperry, T. and P. Thomas. 1999. Identification of two nuclear androgen receptors in kelp bass (Paralabrax clathratus) and their binding affinities for xenobiotics: comparison with Atlantic croaker (Micropogonias undulatus) androgen receptors. Biology of Reproduction 61:1152-1161.
120 Khan, I.A., M.B. Hawkins and P. Thomas. 1999. Gonadal stage-dependent effects of gonadal steroids on gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). Biology of Reproduction 61:834-841.
119 Pinter, J. and P. Thomas. 1999. Induction of ovulation of mature oocytes by the maturation-inducing steroid 17,20β -21-trihydroxy-4-pregnen-3-one in the spotted seatrout. General and Comparative Endocrinology 115:200-209.
118 Loomis, K. and P. Thomas. 1999. Binding characteristics of estrogen receptor (ER) in Atlantic croaker (Micropogonias undulatus) testis: different affinity for estrogens and xenobiotics from that of hepatic ER. Biology of Reproduction 61:51-60.
117 Chang, X., R. Patiño, P. Thomas, and G. Yoshizaki. 1999. Developmental and protein kinase-dependent regulation of ovarian connexin mRNA and oocyte maturational competence in Atlantic croaker. General and Comparative Endocrinology 114(3):330-339.
116 Khan, I.A. and P. Thomas. 1999. GABA exerts stimulatory and inhibitory influences on gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). Neuroendocrinology 69:261-268.
115 Das, S. and P. Thomas. 1999. Pesticides interfere with the nongenomic action of a progestogen on meiotic maturation by binding to its plasma membrane receptor on fish oocytes. Endocrinology 140(4):1953-1956.
114 Sperry, T. and P. Thomas. 1999. Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities. Endocrinology 140(4):1602-1611.
113 Faulk, C.K., L.A. Fuiman and P. Thomas. 1999. Parental exposure to ortho, para-dichlorodiphenyltrichloroethane impairs survival skills of Atlantic croaker (Micropogonias undulatus) larvae. Environmental Toxicology and Chemistry 18(2):254-262.
112 Zhu, Y., Y. Yoshuira, K. Kikuchi, K. Aida, and P. Thomas. 1999. Cloning and phylogenetic relationship of red drum somatolactin cDNA and effects of light on pituitary somatolactin mRNA expression. General and Comparative Endocrinology 113:69-79.
111 Mylonas, C.C, Y. Zohar, L.C. Woods, III, P. Thomas, and R.W. Schulz. 1998. Hormone profiles of captive striped bass Morone saxatilis during spermiation, and long-term enhancement of milt production. Journal of the World Aquaculture Society 29(4):379-392.
110 Thomas, P., D. Breckenridge-Miller and C. Detweiler. 1998. The teleost sperm membrane progestogen receptor: interactions with xenoestrogens. Marine Environmental Research 46:163-167.
109 Khan, I.A. and P. Thomas. 1998. Estradiol-17β and o,p’-DDT stimulate gonadotropin release in Atlantic croaker. Marine Environmental Research 46:149-152.
108 Dunlap, K.D., P. Thomas and H.H. Zakon. 1998. Diversity of sexual dimorphism in electrocommunication signals and its androgen regulation in a genus of electric fish, Apteronotus. Journal of Comparative Physiology 183:77-86.
107 Zhu, Y. and P. Thomas. 1998. Effects of light on plasma somatolactin levels in red drum (Sciaenops ocellatus). General and Comparative Endocrinology 111:76-82.
106 Johnson, A.K., P. Thomas and R.R. Wilson, Jr. 1998. Seasonal cycles of gonadal development and plasma sex steroid levels in Epinephelus morio, a protogynous grouper in the eastern Gulf of Mexico. Journal of Fish Biology 52:502-518.
105 Mylonas, C.C., L.C. Woods, III, P. Thomas, and Y. Zohar. 1998. Endocrine profiles of female striped bass (Morone saxatilis) in captivity, during postvitellogenesis and induction of final oocyte maturation via controlled-release GnRHa-delivery systems. General and Comparative Endocrinology 110:276-289.
104 Detweiler, C. and P. Thomas. 1998. Role of ions and ion channels in the regulation of Atlantic croaker sperm motility. Journal of Experimental Zoology 281:139-148.
103 Thomas, P., D. Breckenridge-Miller and C. Detweiler. 1997. Binding characteristics and regulation of the 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor on testicular and sperm plasma membranes of spotted seatrout (Cynoscion nebulosus). Fish Physiology and Biochemistry 17:109-116.
102 Zhu, Y. and P. Thomas. 1997. Studies on the physiology of somatolactin secretion in red drum and Atlantic croaker. Fish Physiology and Biochemistry 17:271-278.
101 Thomas, P. and S. Das. 1997. Correlation between binding affinities of C21 steroids for the maturation-inducing steroid membrane receptor in spotted seatrout ovaries and their agonist and antagonist activities in an oocyte maturation bioassay. Biology of Reproduction 57:999-1007.
100 Laidley, C.W. and P. Thomas. 1997. Changes in plasma sex binding protein levels associated with ovarian recrudescence in the spotted seatrout (Cynoscion nebulosus). Biology of Reproduction 54(4):931-937.
99 Pinter, J. and P. Thomas. 1997. The ovarian progestogen receptor in the spotted seatrout, Cynoscion nebulosus, demonstrates steroid specificity intermediate between progesterone and glucocorticoid receptors in other vertebrates. Journal of Steroid Biochemistry and Molecular Biology 60(1-2):113-119.
98 Khan, I.A. and P. Thomas. 1997. Aroclor 1254-induced alterations in hypothalamic monoamine metabolism in the Atlantic croaker (Micropogonias undulatus): correlation with pituitary gonadotropin release. NeuroToxicology 18(2):553-560.
97 Zhu, Y. and P. Thomas. 1997. Effects of somatolactin on melanosome aggregation in the melanophores of red drum (Sciaenops ocellatus) scales. General and Comparative Endocrinology 105:127-133.
96 King, V, W., S. Ghosh, P. Thomas, and C.V. Sullivan. 1997. A receptor for the oocyte maturation-inducing hormone, 17,20β,21-trihydroxy-4-pregnen-3-one on ovarian membranes of striped bass. Biology of Reproduction 56:266-271.
95 Ungerer, J. and P. Thomas. 1996. Role of the very low density lipoproteins in the accumulation of o,p’-DDT in fish ovaries during gonadal recrudescence. Aquatic Toxicology 35(3,4):183-195.
94 Khan, I.A. and P. Thomas. 1996. Melatonin influences gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). General and Comparative Endocrinology 104:231-242.
93 Khan, I. and P. Thomas. 1996. Disruption of neuroendocrine function in Atlantic croaker exposed to Aroclor 1254. Marine Environmental Research 42:145-149.
92 Ungerer, J. and P. Thomas. 1996. Transport and accumulation of organochlorines in the ovaries of Atlantic croaker (Micropogonias undulatus). Marine Environmental Research 42:167-171.
91 Zhu, Y. and P. Thomas. 1996. Elevations of somatolactin in plasma and pituitaries and increased -MSH cell activity in red drum exposed to black background and decreased illumination. General and Comparative Endocrinology 101:21-31.
90 Zhu, Y. and P. Thomas. 1995. Red drum somatolactin: development of a homologous radioimmunoassay and plasma levels after exposure to stressors or various backgrounds. General and Comparative Endocrinology 99:275-288.
89 Pinter, J. and P. Thomas. 1995. Characterization of a progestogen receptor in the ovary of the spotted seatrout, Cynoscion nebulosus. Biology of Reproduction 52:667-675.
88 Thomas, P. and L. Budiantara. 1995. Reproductive life history stages sensitive to oil and naphthalene in Atlantic croaker. Marine Environmental Research 39:147-150.
87 Ghosh, S. and P. Thomas. 1995. Antagonistic effects of xenobiotics on steroid-induced final maturation of Atlantic croaker oocytes in vitro. Marine Environmental Research 39:159-163.
86 Ghosh, P. and P. Thomas. 1995. Binding of metals to red drum vitellogenin and incorporation into oocytes. Marine Environmental Research 39:165-168.
85 Laidley, C.W. and P. Thomas. 1994. Partial characterization of a sex-steroid binding protein in the spotted seatrout (Cynoscion nebulosus). Biology of Reproduction 51:982-992.
84 Thomas, P., P.A. Copeland and J.A. Prentice. 1994. Preliminary observations on the reproductive physiology of female orangemouth corvina in captivity. Journal of the World Aquaculture Society 25(2):214-224.
83 King, W.V., P. Thomas and C.V. Sullivan. 1994. Hormonal regulation of final maturation of striped bass oocytes in vitro. General and Comparative Endocrinology 96:223-233.
82 Thomas, P., K.N. Baer and R.B. White. 1994. Isolation and partial characterization of metallothionein in the liver of the red-eared turtle (Trachemys scripta) following intraperitoneal administration of cadmium. Comparative Biochemistry and Physiology, Part C 107:221-226.
81 King, William V., P. Thomas, R.M. Harrell, R.G. Hodson, and C.V. Sullivan. 1994. Plasma levels of gonadal steroids during final oocyte maturation of striped bass, Morone saxatilis L. General and Comparative Endocrinology 95:178-191.
80 Khan, I.A. and P. Thomas. 1994. Seasonal and daily variations in the plasma gonadotropin II response to a LHRH analog and serotonin in Atlantic croaker (Micropogonias undulatus): Evidence for mediation by 5-HT2 receptors. The Journal of Experimental Zoology 269:531-537.
79 Yoshizaki, G., R. Patiño and P. Thomas. 1994. Connexin messenger ribonucleic acids in the ovary of Atlantic croaker: Molecular cloning and characterization, hormonal control, and correlation with appearance of oocyte maturational competence. Biology of Reproduction 51:493-503.
78 Barry, T.P., P. Thomas and G.V. Callard. 1993. Stage-related production of 21-hydroxylated progestins by the dogfish (Squalus acanthias) testis. Journal of Experimental Zoology 265:522-532.
77 Copeland, P.A. and P. Thomas. 1993. Isolation of gonadotropin subunits and evidence for two distinct gonadotropins in Atlantic croaker (Micropogonias undulatus). General and Comparative Endocrinology 91:115-125.
76 Godwin, J.R. and P. Thomas. 1993. Sex change and steroid profiles in the protandrous anemonefish Amphiprion melanopus (Pomacentridae, Teleostei). General and Comparative Endocrinology 91:144-157.
75 Khan, I.A. and P. Thomas. 1993. Immunocytochemical localization of serotonin and gonadotropin-releasing hormone in the brain and pituitary gland of the Atlantic croaker, Micropogonias undulatus. General and Comparative Endocrinology 91:167-180.
74 Singh, H. and P. Thomas. 1993. Mechanism of stimulatory action of growth hormone on ovarian steroidogenesis in spotted seatrout, Cynoscion nebulosus. General and Comparative Endocrinology 89:341-353.
73 Thomas, P. 1993. Effects of cadmium on gonadotropin secretion from Atlantic croaker pituitaries incubated in vitro. Marine Environmental Research 35:141-145.
72 Thomas, P. and J. Smith. 1993. Binding of xenobiotics to the estrogen receptor of spotted seatrout: A screening assay for potential estrogenic effects. Marine Environmental Research 35:147-151.
71 Thomas, P. and H.W. Wofford. 1993. Effects of cadmium and Aroclor 1254 on lipid peroxidation, glutathione peroxidase activity, and selected antioxidants in Atlantic croaker tissues. Aquatic Toxicology 27:159-178.
70 York, W.S., R. Patiño and P. Thomas. 1993. Ultrastructural changes in follicle cell-oocyte associations during development and maturation of the ovarian follicle in Atlantic croaker. General and Comparative Endocrinology 92:402-418.
69 Cameron, J.N. and P. Thomas. 1992. Calcitonin-like immunoreactivity in the blue crab: tissue distribution, variations in the molt cycle, and partial characterization. Journal of Experimental Zoology 262:279-286.
68 Copeland, P.A. and P. Thomas. 1992. Isolation of maturational gonadotropin subunits from spotted seatrout (Cynoscion nebulosus) and development of a beta subunit-directed radioimmunoassay for gonadotropin measurement in sciaenid fish. General and Comparative Endocrinology 88:100-110.
67 Khan, I.A. and P. Thomas. 1992. Stimulatory effects of serotonin on maturational gonadotropin release in the Atlantic croaker, (Micropogonias undulatus). General and Comparative Endocrinology 88:388-396.
66 Thomas, P. and M.J. Juedes. 1992. Influence of lead on the glutathione status of Atlantic croaker tissues. Aquatic Toxicology 23:11-30.
65 White, R.B. and P. Thomas. 1992. Adrenal-kidney and gonadal steroidogenesis during sexual differentiation of a reptile with temperature-dependent sex determination. General and Comparative Endocrinology 88:10-19.
64 White, R.B. and P. Thomas. 1992. Stimulation of in vitro steroidogenesis by pituitary hormones in a turtle (Trachemys scripta) within the temperature-sensitive period for sex determination. Biology of Reproduction 47:952-959.
63 White, R.B. and P. Thomas. 1992. Whole-body and plasma concentrations of steroids in the turtle, Trachemys scripta, before, during, and after the temperature-sensitive period for sex determination. Journal of Experimental Zoology 264:159-166.
62 Baer, K.N. and P. Thomas. 1991. Isolation of novel metal-binding proteins distinct from metallothionein from spotted seatrout (Cynoscion nebulosus) and Atlantic croaker (Micropogonias undulatus) ovaries. Marine Biology 108:31-37.
61 LaFleur, G.L. and P. Thomas. 1991. Evidence for a role of Na+, K+ - ATPase in the hydration of Atlantic croaker and spotted seatrout oocytes during final maturation. Journal Experimental Zoology 258:126-136.
60 Smith, J.S. and P. Thomas. 1991. Changes in hepatic estrogen-receptor concentrations during the annual reproductive and ovarian cycles of a marine teleost, the spotted seatrout Cynoscion nebulosus. General and Comparative Endocrinology 81:234-245.
59 Thomas, P. and L. Robertson. 1991. Plasma cortisol and glucose stress responses of red drum (Sciaenops ocellatus) to handling and shallow water stressors and anesthesia with MS 222, quinaldine sulfate and metomidate. Aquaculture 96:69-86.
58 Yan, H.Y. and P. Thomas. 1991. Histochemical and immunocytochemical identification of the pituitary cell types in three sciaenid fishes: Atlantic croaker (Micropogonias undulatus), spotted seatrout (Cynoscion nebulosus) and red drum (Sciaenops ocellatus). General and Comparative Endocrinology 84:389-400.
57 Zakon, H.H., P. Thomas and H-Y. Hong. 1991. Electric organ discharge frequencies and plasma sex steroid levels during gonadal recrudescence in a natural population of Sternopygus macrurus. Journal Comparative Physiology 169:492-499.
56 Baer, K.N. and P. Thomas. 1990. Influence of capture stress, salinity and reproductive status on zinc associated with metallothionein-like proteins in the livers of three marine teleost species. Marine Environmental Research 29:277-287.
55 Landsman, R.E., C.F. Harding, P. Moller, and P. Thomas. 1990. The effects of androgens and estrogen on the external morphology and electric organ discharge waveform of Gnathonemus petersii (Mormyridae, Teleostei). Hormones and Behavior 24:532-553.
54 Pajor, A.M., J.J. Stegeman, P. Thomas, and B.R. Woodin. 1990. Feminization of the hepatic microsomal cytochrome P-450 system in brook trout by estradiol, testosterone and pituitary factors. Journal of Experimental Zoology 253: 51-60.
53 Patiño, R. and P. Thomas. 1990. Characterization of membrane receptor activity for 17,20β,21-trihydroxy-4-pregnen-3-one in ovaries of spotted seatrout (Cynoscion nebulosus). General and Comparative Endocrinology 78:204-217.
52 Patiño, R. and P. Thomas. 1990. Effects of gonadotropin on ovarian intrafollicular processes during the development of oocytes maturational competence in a teleost, the Atlantic croaker. Evidence for two distinct stages of gonadotropic control of final oocytes maturation. Biology of Reproduction 43:818-827.
51 Patiño, R. and P. Thomas. 1990. Gonadotropin stimulates 17,20 β,21-trihydroxy-4-pregnen-3-one production from endogenous substrates in Atlantic croaker ovarian follicles undergoing final maturation in vitro. General and Comparative Endocrinology 78:474-478.
50 Patiño, R. and P. Thomas. 1990. Induction of maturation of Atlantic croaker oocytes by 17,20β, 21-trihydroxy-4-pregnen-3-one in vitro: Consideration of some biological and experimental variables. Journal of Experimental Zoology 255:97-109.
49 Smith, J.S. and P. Thomas. 1990. Binding characteristics of the hepatic estrogen receptor of the spotted seatrout, Cynoscion nebulosus. General and Comparative Endocrinology 77:29 42.
48 Thomas, P. 1990. Teleost model for studying the effects of chemicals on female reproductive endocrine function. Journal of Experimental Zoology (Supplement 4):126-128.
47 Zakon, H.H., H.Y. Yan, and P. Thomas. 1990. Human chorionic gonadotropin-induced shifts in the electrosensory and motor systems of the weakly electric fish Sternopygus. Journal of Neurobiology 21:826-833.
46 Baer, K.N. and P. Thomas. 1989. Purification and isolation of a non-metallothionein Zn-binding protein from spotted seatrout (Cynoscion nebulosus) oocytes. Marine Environmental Research 28:157-161.
45 Copeland, P.A. and P. Thomas. 1989. Control of gonadotropin release in the Atlantic croaker: evidence for lack of dopaminergic inhibition. General and Comparative Endocrinology 74:474 483.
44 Copeland, P.A. and P. Thomas. 1989. Purification of maturational gonadotropin from Atlantic croaker (Micropogonias undulatus) and development of a homologous radioimmunoassay. General and Comparative Endocrinology 73:425-441.
43 MacKenzie, D.S., P. Thomas and S.M. Farrar. 1989. Seasonal changes in thyroid and reproductive steroid hormones in female channel catfish (Ictalurus punctatus) in pond culture. Aquaculture 78:63-80.
42 Thomas, P. 1989. Effects of Aroclor 1254 and cadmium on reproductive endocrine function and ovarian growth in Atlantic croaker. Marine Environmental Research 28:499-503.
41 Thomas, P. and N.W. Boyd. 1989. Dietary administration of a LHRH analog induces successful spawning of spotted seatrout (Cynoscion nebulosus). Aquaculture 80:363-370.
40 Thomas, P. and J.M. Trant. 1989. Evidence that 17,20β,21-trihydroxy-4-pregnen-3-one is a maturation-inducing steroid in spotted seatrout. Fish Physiology and Biochemistry 7:185 191.
39 Trant, J.M. and P. Thomas. 1989. Changes in ovarian steroidogenesis in vitro associated with final maturation of Atlantic croaker oocytes. General and Comparative Endocrinology 75:405-412.
38 Trant, J.M. and P. Thomas. 1989. Isolation of a novel maturation-inducing steroid produced in vitro by ovaries of Atlantic croaker. General and Comparative Endocrinology 75:397 404.
37 Brown-Peterson, N. and P. Thomas. 1988. Differing reproductive characteristics of temperate and subtropical groups of Cynoscion nebulosus. Contributions in Marine Science 30 (Suppl.):71-77.
36 Brown-Peterson, N., P. Thomas and C.R. Arnold. 1988. Reproductive biology of the spotted seatrout, Cynoscion nebulosus, in South Texas. Fisheries Bulletin 86(2):373-388.
35 Copeland, P.A. and P. Thomas. 1988. The measurement of plasma vitellogenin levels in a marine teleost, the spotted seatrout (Cynoscion nebulosus) by homologous radioimmunoassay. Comparative Biochemistry and Physiology B 91(1):17-23.
34 Lewis, D.H. and P. Thomas. 1988. Assessing immunocompetence of red drum. Contributions in Marine Science 30 (Suppl.):153-156.
33 Robertson, L., P. Thomas and C.R. Arnold. 1988. Plasma cortisol and secondary stress responses of cultured red drum (Sciaenops ocellatus) to several transportation procedures. Aquaculture 68:115-130.
32 Singh, H., R.W. Griffith, A. Takahashi, H. Kawauchi, P. Thomas, and J.J. Stegeman. 1988. Regulation of gonadal steroidogenesis in Fundulus heteroclitus by recombinant salmon growth hormone. General and Comparative Endocrinology 72:144-153.
31 Sorensen, E.M.B. and P. Thomas. 1988. Selenium accumulation, reproductive status, and histopathological changes in environmentally exposed redear sunfish. Archives of Toxicology 61:324-329.
30 Thomas, P. 1988. Reproductive endocrine function in female Atlantic croaker exposed to xenobiotics. Marine Environmental Research 24:179-183.
29 Thomas, P. and N. Boyd. 1988. Induced spawning of spotted seatrout, red drum and orangemouth corvina (family: Sciaenidae) with luteinizing hormone-releasing hormone analog injection. Contributions in Marine Science 30 (Suppl.):43-47.
28 Thomas, P., M. Westerman, P.F. Dehn, E. Nowicki, G.J. Holt, and C.R. Arnold. 1988. Growth of juvenile red drum: adenylate metabolism, RNA-DNA ratio and effects of growth hormone. Contributions in Marine Science 30 (Suppl.):29-35.
27 Trant, J.M. and P. Thomas. 1988. Structure-activity relationships of steroids in inducing germinal vesicle breakdown of Atlantic croaker oocytes in vitro. General and Comparative Endocrinology 71:307-317.
26 Wofford, H.W. and P. Thomas. 1988. Effect of xenobiotics on peroxidation of microsomal lipids from striped mullet (Mugilcephalus) and Atlantic croaker (Micropogonias undulatus). Marine Environmental Research 24:285-2889.
25 Wofford, H.W. and P. Thomas. 1988. Peroxidation of mullet and rat liver lipids in vitro: Effects of pyridine nucleotides, iron, incubation buffer, and xenobiotics. Comparative Biochemistry Physiology 89C(2):201-205.
24 Yan, H.Y. and P. Thomas. 1988. Changes in prolactin cell size and chloride cell number in young red drum (Sciaenops ocellatus) during salinity adaptation. Contributions in Marine Science 30 (Suppl.):157-164.
23 Prentice, J.A. and P. Thomas. 1987. Successful spawning of orangemouth corvina following des-Gly10, [D-Ala6]-luteinizing hormone-releasing hormone (1-9) ethylamide and pimozide injection. Progressive Fish Culturist 49:66-69.
22 Robertson, L., P. Thomas, C.R. Arnold, and J.M. Trant. 1987. Plasma cortisol and secondary stress responses of red drum (Sciaenops ocellatus) to handling, transport, rearing density and an outbreak of disease. Progressive Fish Culturist 49:1-12.
21 Thomas, P. 1987. Influence of some environmental variables on the ascorbic acid status of mullet, (Mugil cephalus) Linn, tissues. III. Effects of exposure to oil. Journal of Fish Biology 30:485-494.
20 Thomas, P. and D.H. Lewis. 1987. Effect of cortisol on immunity in red drum, (Sciaenops ocellatus). Journal of Fish Biology 31(Suppl. A):123-127.
19 Trant, J.M., P. Thomas and C.H.L. Shackleton. 1986. Identification of 17,20β,21-trihydroxy-4-pregnen-3-one as the major ovarian steroid produced by the teleost (Micropogonias undulatus) during final oocyte maturation. Steroids 47:89-99.
18 Steele, C.W., D.W.M. Owens, A.D. Scarfe, and P. Thomas. 1985. Behavioral assessment of the sublethal effects of aquatic pollutants. Marine Pollution Bulletin 16(6):221-224.
17 Thomas, P., M.B. Bally and J.M. Neff. 1985. Influence of some environmental variables on the ascorbic acid status of mullet (Mugilcephalus Linn.) tissues. II. Seasonal fluctuations and biosynthetic ability. Journal of Fish Biology 27:47-57.
16 Thomas, P. and M.J. Juedes. 1985. Altered glutathione status in Atlantic croaker (Micropogonias undulatus) exposed to lead. Marine Environmental Research 17:192 195.
15 Wofford, H.W. and P. Thomas. 1985. Comparison of microsomal lipid peroxidation in fish and rats. Marine Environmental Research 17:215-217.
14 Thomas, P. 1984. Influence of some environmental variables on the ascorbic acid status of mullet (Mugil cephalus Linn.) tissues: 1. Effect of salinity, capture-stress and temperature. Journal of Fish Biology 25:711-720.
13 Thomas, P. and J.M. Neff. 1984. Effects of a pollutant and other environmental variables on the ascorbic acid content of fish tissues. Marine Environmental Research 14:489-491.
12 Thomas, P. and H.W. Wofford. 1984. Effects of metals and organic compounds on hepatic glutathione, cysteine and acid soluble thiol levels in mullet (Mugil cephalus L.). Toxicology Applied Pharmacology 76:172-182.
11 Thomas, P. and H.W. Wofford. 1984. Elevated acid soluble thiol content in fish hepatic tissue: a response to pollutants. Marine Environmental Research 14:486-488.
10 Thomas, P. and H.W. Wofford. 1984. High-performance liquid chromatography of corticosteroids in vertebrate plasma: assay of cortisol in mullet and corticosterone in the rat. Comparative Biochemistry and Physiology 78(2):473-479.
9 Wofford, H.W. and P. Thomas. 1984. Interactions of cadmium with sulfhydryl-containing compounds in striped mullet (Mugilcephalus L.). Marine Environmental Research 14:119-137.
8 Carr, R.S., M.B. Bally, P. Thomas, and J.M. Neff. 1983. Comparison of methods for the determination of ascorbic acid in animal tissues. Analytical Chemistry 55:1229-1232.
7 Kamstra, G.K., P. Thomas and Janet Sadow. 1983. Evaluation of changes in the secretion of corticotrophin releasing activity using the isolated rat hypothalamus incubated in vitro. Journal of Endocrinology 97:291-300.
6 Carr, R.S., P. Thomas and J.M. Neff. 1982. A simple spectrophotometric technique for the determination of pentachlorophenol in water. Bulletin Environmental Contamination and Toxicology 28:477-479.
5 Stegeman, J.J., A.M. Pajor and P. Thomas. 1982. Influence of estradiol and testosterone on cytochrome P-450 and monooxygenase activity in immature brook trout (Salvelinus fontinalis). Biochemical Pharmacology 31:3979-3989.
4 Thomas, P., M. Bally and J.M. Neff. 1982. Ascorbic acid status of mullet (Mugil cephalus Linn.) exposed to cadmium. Journal of Fish Biology 20:183-196.
3 Thomas, P., H.W. Wofford and J.M. Neff. 1981. Biochemical stress responses of striped mullet (Mugil cephalus L.) to fluorene analogs. Aquatic Toxicology: 329-342.
2 Thomas, P., B. Woodin and J.M. Neff. 1980. Biochemical responses of striped mullet (Mugil cephalus) to oil exposure. I. Acute responses-interrenal activation and secondary stress responses. Marine Biology 59:141-149.
1 Ishii, S., P. Thomas and S. Nakamura. 1973. Morphometric classification of the neurosecretory granules in the rat pars nervosa. Z. Zellforsch 146:463-471.
BOOK CHAPTERS
29
Thomas, P. 2008. The Endocrine System. In: Toxicology of Fishes. R. Di Giulio and D.E. Hinton (eds.), Chapter 10, pp 457-488. Taylor & Francis: Boca Raton, FL.
28 Thomas, P., C. Tubbs, H. Berg, and G. Dressing. 2007. Sex steroid hormone receptors in fish ovaries. In: The Fish Oocyte: From Basic Studies to Biotechnological Applications. P.J. Babin, J. Cerda and E. Lubzens (eds.), Chapter 8, pp 203-234. Springer, Dordrecht, Netherlands.
27 Thomas, P. and I.A. Khan. 2005. Disruption of nongenomic steroid actions on gametes and serotonergic pathways controlling reproductive neuroendocrine function by environmental chemicals. In: Endocrine Disruptors: Effects on Male and Female Reproductive Systems. R.K. Nas (ed.), Chapter 1, pp. 3-45. CRC Press, Boca Raton, Florida.
26 Thomas, P., Y. Zhu and Y. Pang. 2003. Current knowledge of the nature and identity of progestin and estrogen membrane receptors in fish gonads. In: The Identities of Membrane Steroid Receptors. C.S. Watson (ed.), pp. 131-138. Boston: Kluwer Academic Publishers.
25 Thomas, P. 2000. Nuclear and membrane steroid receptors and their functions in teleost gonads. pp. 149-156. In: Proceedings of the Sixth International Symposium of the Reproductive Physiology of Fish. B. Norberg, O.S. Kjesbu, G.L. Taranger, E. Andersson and S.O. Stephensson (eds), July 3-8, 1999, Bergen, Norway.
24 Thomas, P. 2000. Disruption of the endocrine control of final oocyte maturation in teleosts by xenobiotic chemicals. In: Environmental Toxicology and Risk Assessment: Eighth Volume. D. Henshel, M.C. Black and M.C. Harrass (eds.), pp. 165-181. ASTM STP 1364, American Society for Testing and Materials, West Conshohocken, PA.
23 Thomas, P. 1999. Nontraditional sites of endocrine disruption by chemicals on the hypothalamus-pituitary gonadal axis: interactions with steroid membrane receptors, monoaminergic pathways and signal transduction systems. In: Endocrine Disruptors: Effects on Male and Female Reproductive Systems. R.K. Naz (ed.), pp. 3-38. CRC Press, Boca Raton, Florida.
22 Khan, I. and P. Thomas. 1999. Fish, female reproductive cycle. In: The Encyclopedia of Reproduction. E. Knobil and J. Neill (eds.), Vol. 3, pp. 552-564. Academic Press.
21 McNabb, A.C., C. Schreck, C. Tyler, P. Thomas, V. Kramer, J. Specker, M. Mayes, and K. Selcer. 1999. Basic Physiology. In: Reproductive and Developmental Effects of Contaminants in Oviparous Vertebrates. R.T. Di Guilio and D.E. Tillet (eds.), pp. 113-224. SETAC Press, Pensacola, Florida.
20 Thomas, P., et al. (contributing author). 1998. Effects of organochlorine chemicals on organisms. In: Ecotoxicological Risk Assessment of the Chlorinated Organic Chemicals. J. Carey, P. Cook, J. Giesy, P. Hodson, D. Muir, W. Owens, and K. Solomon (eds.), pp. 107-140. SETAC Press, Pensacola, Florida.
19 Ankley, G., et al. (P. Thomas). 1998. Overview of a workshop on screening methods for detecting potential (anti-) estrogenic/androgenic chemicals in wildlife. Environmental Toxicology and Chemistry 17:67-87.
18 Khan, I.A. and P. Thomas. 1999. Ovarian cycle, teleost fish. In: Encyclopedia of Reproduction. E. Knobil and J.D. Neill (eds.), Volume 3, pp. 552-564. Academic Press, San Diego.
17 Thomas, P. and I.A. Khan. 1997. Mechanisms of chemical interference with reproductive endocrine function in sciaenid fishes. In: Chemically Induced Alterations in Functional Development and Reproduction of Fishes. R.M. Rolland, M. Gilbertson, and R.E. Peterson (eds.), pp. 29-51. SETAC Technical Publications Series.
16 Spies, R.B. and P. Thomas. 1997. Reproductive and endocrine status of female kelp bass from a contaminated site in the Southern California Bight and estrogen receptor binding of DDTs. In: Chemically Induced Alterations in Functional Development and Reproduction of Fishes. R.M. Rolland, M. Gilbertson, and R.E. Peterson (eds.), pp. 113-133. SETAC Technical Publications Series.
15 Thomas, P., S. Das, D. Breckenridge-Miller, and C. Detweiler. 1997. Characterization and regulation of a progestin receptor on Atlantic croaker sperm membranes. In: Advances in Comparative Endocrinology, Volume II. S. Kawashima and S. Kikuyama (eds.), pp. 1381-1385. Monduzzi Editore S.p.A.
14 Pinter, J. and P. Thomas. 1995. Studies of the nuclear progestogen receptor in the ovary of the spotted seatrout: regulation of the final stages of ovarian development. In: Proceedings of the Fifth International Symposium on the Reproductive Physiology of Fish. F.W. Goetz and P. Thomas (eds.), pp. 302-304. The University of Texas at Austin, Printing Department, Austin, TX, USA.
13 Thomas, P., C.R. Arnold, and G.J. Holt. 1995. Sciaenid fishes. In: Broodstock Management and Egg and Larval Quality. N. Bromage and R.J. Roberts (eds.), pp. 118-137. Blackwell Scientific Publishers.
12 Yoshizaki, G., W. Jin, R. Patiño, and P. Thomas. 1995. Connexin genes, gap junctions, and ovarian maturational competence. In: Proceedings of the Fifth International Symposium on the Reproductive Physiology of Fish. F.W. Goetz and P. Thomas (eds.), pp. 342-344. The University of Texas at Austin, Printing Department, Austin, TX, USA.
11 Thomas, P. 1994. Hormonal control of final oocyte maturation in sciaenid fishes. K.G. Davey, R.E. Peter, and S.S. Tobe (eds.), pp. 619-625. Nat. Res. Council of Canada, Ottawa.
10 Thomas, P. and C.R. Arnold. 1993. Environmental and hormonal control of reproduction in sciaenid fish. In: Recent Advances in Aquaculture. J.F. Miur and R.J. Roberts (eds.), pp. 31-42. Blackwell Scientific Publications, Oxford
9 Thomas, P., R.S. Carr and J.M. Neff. 1987. Biochemical responses and alterations of tissue ascorbic acid and glutathione content. In: Pollutant Studies in Marine Animals. C.S. Giam and L.E. Ray (eds.), pp. 155-180. CRC Press.
8 Spies, R.B., J.J. Stegeman, D.W. Rice, Jr., B. Woodin, P. Thomas, J.E. Hose, J.N. Cross, and M. Prieto. 1991. Sublethal responses of Platichthys stellatus to organic contamination in San Francisco Bay with emphasis on reproduction. In: Biomarkers of Environmental Contamination, pp. 87-122. Lewis Publishers, Inc.
7 Thomas, P. and R. Patiño. 1991. Changes 17,20β,21-trihydroxy-4-pregnen-3-one membrane receptor concentrations in ovaries of spotted seatrout during final oocyte maturation. In: Proceedings 4th International Symposium on Reproductive Physiology of Fish. A.P. Scott, J. Sumpter, D. Kime and M.S. Rolge (eds.), pp. 122-124. University of East Anglia.
6 Thomas, P. 1990. Biochemical and molecular responses of fish to stressors and their potential use in environmental monitoring. In: Transactions of the American Fisheries Society, Symposium 8:9-28.
5 Giam, C.S., L.E. Ray, R.S. Anderson, C.R. Fries, R. Lee, J.M. Neff, J.J. Stegeman, P. Thomas, and M.R. Tripp. 1987. Pollutant Responses in Marine Animals. The Program. In: Pollutant Studies in Marine Animals. C.S. Giam and L.E. Ray (eds.), pp. 1-21. CRC Press.
4 Thomas, P., R.S. Carr and J.M. Neff. 1987. Biochemical responses and alterations of tissue ascorbic acid and glutathione content. In: Pollutant Studies in Marine Animals. C.S. Giam and L.E. Ray (eds.), pp. 155-180. CRC Press.
3 Thomas, P. and J.M. Neff. 1985. Plasma corticosteroid and glucose responses to pollutants in striped mullet: different effects of naphthalene, benzo[a]pyrene and cadmium exposure. In: Marine Pollution and Physiology: Recent Advances. A. Calabrese, F.P. Thurberg, F.J. Vernberg, and W.B. Vernberg (eds.), pp. 63-82. University of South Carolina Press.
2 Thomas, P., H.W. Wofford and J.M. Neff. 1982. Effect of cadmium on glutathione content of mullet (Mugil cephalus) tissues. In: Pollution and Physiology of Marine Organisms. A. Calabrese, F.P. Thurberg, F.J. Vernberg, and W.B. Vernberg (eds.), pp. 109-125. Academic Press, N.Y.
1 Thomas, P., R.S. Carr and J.M. Neff. 1981. Biochemical responses of mullet (Mugil cephalus) and polychaete worms Nereis virens to pentachlorophenol. In: Monitoring of Marine Pollutants. A. Calabrese, F.P. Thurberg, F.J. Vernberg and W.B. Vernberg (eds.), pp.73-103. Biological. Academic Press, N.Y.
-
HONORS AND AWARDS
2008 Howard Bern Distinguished Lecture in Comparative Endocrinology, Division of Comparative Endocrinology of the Society of Integrative and Comparative Biology Annual Meeting. 2006 Irving I. Geswhind Memorial Lecture in Comparative Endocrinology, Western Regional Division of Comparative Endocrinology of the Society of Integrative Biology. 2006 Honorary Doctor of Philosophy in Biology from Örebro University, Sweden 2003 H.E.B. Endowed Chair in Marine Science, The University of Texas at Austin, 1972 Rotary Club of Japan Research Scholarship (2yrs)
-
Current Staff


Yefei Pang, Ph.D.
Research ScientistSaydur Rahman, Ph.D.
Research Scientist

Jing Dong
Research Scientist AssistantSusan Lawson
Laboratory Research AssistantCurrent Graduate Students


Aubrey Converse
Ph.D. StudentKathryn Ondricek
Master's StudentRecent Graduates

Amanda Fitzgerald
Master's StudentFormer Students
Wenxian Tan, Ph.D., Fall 2013
Chenan Zhang, M.S., Fall 2011
Candace Peyton, Masters, Spring 2009
Gwen E. Dressing, Ph.D., Spring 2008
Current Position: Postdoctoral Fellow, University of Minnesota Cancer Center
Christopher W. Tubbs, Ph.D., Summer 2007
Current Position: Postdoctoral Fellow, San Diego Zoo Wildlife Park
Caleb Harris, Masters, Fall 2007
Margaret (Sam) Pace, Ph.D., Spring 2005
Current position: Postdoctoral Fellow, Baylor College of Medicine
Abby D. Benninghoff, Ph.D., Summer 2004
Current position: Postdoctoral Fellow, Oregon State University
Alyssa M. Braun, Ph.D., Spring 2002
Current position: Visiting Assistant Professor/Postdoctoral Fellow, University of Nevada, Las Vegas
Mary Beth Hawkins, Ph.D., Summer 2002
Current position: Assistant Professor, North Carolina State University
C. LaSonya Mathews, M.S., Spring 2001
Karen Rogowski, M.A., Fall 1999
Todd Sperry, Ph.D., Spring 1999
A. Katrina Loomis, M.A., Fall 1998
Charles Detweiler, M.A., Fall 1997
Jonathon Pinter, Ph.D., Summer 1995
Charles Laidley, Ph.D., Spring 1995
Lestarini Budiantara, M.A., Spring 1994
Jennifer Di Cocco, M.A., Summer 1994
Susan Safford, Ph.D., Spring 1992 (Dr. Clark Hubbs co-supervisor)
Richard White, Ph.D., 1991 (Dr. Jim Bull co-supervisor)
John Smith, Ph.D., Spring 1990 (Dr. David Crews co-supervisor)
Elizabeth Sharpe, M.A., 1989 Texas A&M University (Dr. Don Lewis co-supervisor)
Gary LaFleur, M.A., 1989 Corpus Christi State University
John Trant, Ph.D., Summer 1987 (Dr. David Crews co-supervisor)
Lori Robertson, M.A., Fall 1984
Marlene Juedes, M.A., 1986 Corpus Christi State University
-














