-

-
Tanya T Paull
Professor, Professor of Oncology
Molecular Biosciences, Department of Oncology
Burl G. and Lorene L. Rogers Chair in Human Health (Holder)Regulation of DNA double-strand break repair and oxidative stress signalingtpaull@utexas.edu
Phone: 512-232-7802
Office Location
MBB 2.448
Postal Address
2500 SPEEDWAY
AUSTIN, TX 78712-
Dr. Paull received her B.S. and M.S. in Biological Sciences from Stanford Univ. in 1991, and received her Ph.D. from UCLA in 1996. Her post-doctoral research with Dr. Martin Gellert at NIH was supported by a fellowship from the Helen Hay Whitney Foundation. Dr. Paull established an independent laboratory in 2000 in the Dept. of Molecular Genetics and Microbiology at the University of Texas at Austin. Her research is aimed toward understanding the mechanisms of DNA double-strand break repair in eukaryotes, as well as the intersection between DNA damage, oxidative stress signaling, and protein homeostasis in human cells. This investigation is relevant to cancer biology and etiology as well as the origins of neurodegeneration in the human population. She was an Investigator with the Howard Hughes Medical Institute from 2008 through 2019 and is currently the Burl and Lorene Rogers Chair in Human Health and Professor in the Department of Molecular Biosciences.
-
Research Summary:
DNA double-strand break repair
Simultaneous breaks in both strands of the DNA duplex are potentially lethal events that can lead to permanent loss of genetic information as well as many different types of misrepair products. These types of events can act as initiating lesions in cancer and can also generate recombination products that determine the outcome of cancer progression. We are interested in the mechanisms underlying the repair of DNA double-strand breaks, particularly the initial events that occur at a break site within a few seconds to a few minutes of break formation. One of the key regulators that binds to double-strand breaks and coordinates its repair is the Mre11/Rad50/Nbs1 (MRN) complex, an ancient molecular machine that processes DNA ends, pairs the broken ends together, and participates in signaling the presence of breaks within cells (1–6). We study the activities of recombinant MRN complexes in eukaryotic cells and also in vitro to characterize its actions on different types of DNA substrates and recombination intermediates.
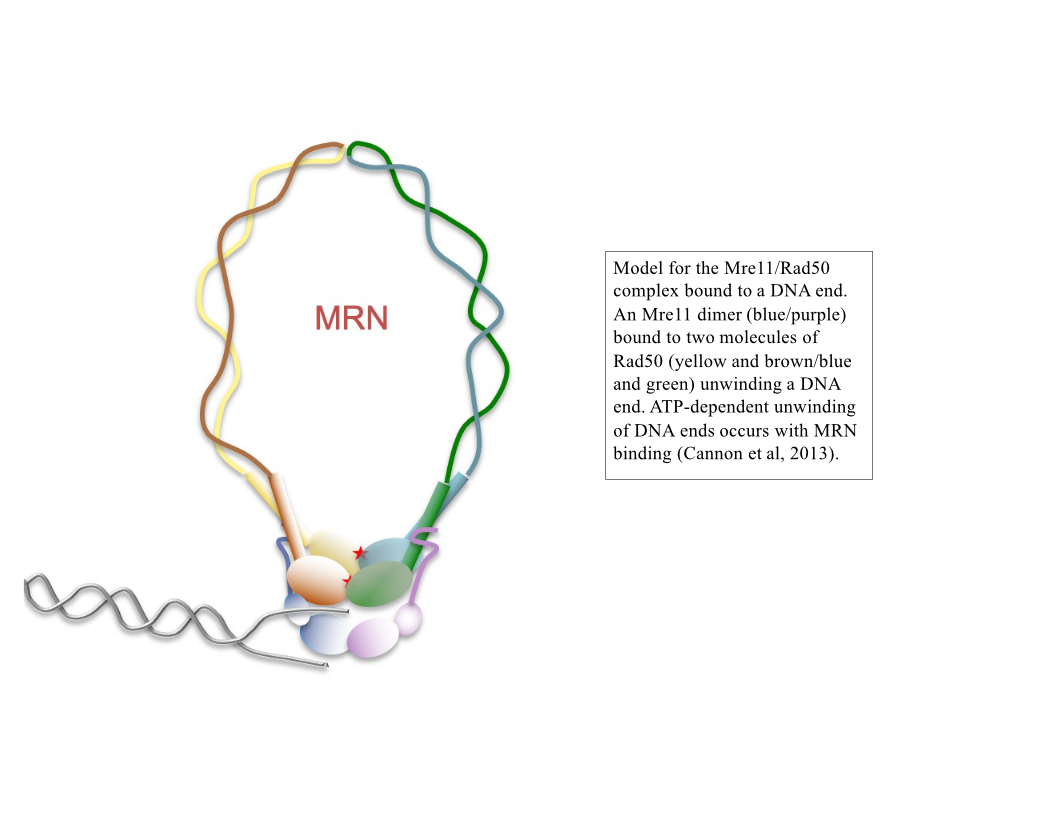
DNA end resection, the removal of hundreds of nucleotides from the 5' strand at double-strand breaks, is an essential part of repair that promotes homologous recombination. Through our work over the past several years we have shown that the MRN complex plays a number of important roles in this process, including the initial short-range resection of the 5ʹ strand by Mre11, recruitment of long-range 5ʹ to 3ʹ nucleases, and displacement/removal of the Ku heterodimer that also binds rapidly to DNA ends and promotes non-homologous end joining (4, 7–9).
Although homologous recombination and non-homologous end joining have long been thought to be competing pathways of DNA repair, our recent results show that the MRN complex works cooperatively with Ku and its binding partner DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to recognize and process ends. This sequential model of DNA end processing is consistent with our results and that of many groups in the field and suggests that non-homologous end joining is attempted first, followed by end processing for homologous recombination (10). Recent work from the lab has also established a novel chromatin immunoprecipitation method to isolate the DNA fragments generated by MRN at DNA-PK-bound sites in mammalian cells, which we have used to validate and extend our in vitro findings (9). Current studies are investigating the patterns, regulation, and cell cycle specificity of these processing events and how MRN-dependent DNA processing occurs in human cells during physiologically relevant DNA damage exposure.
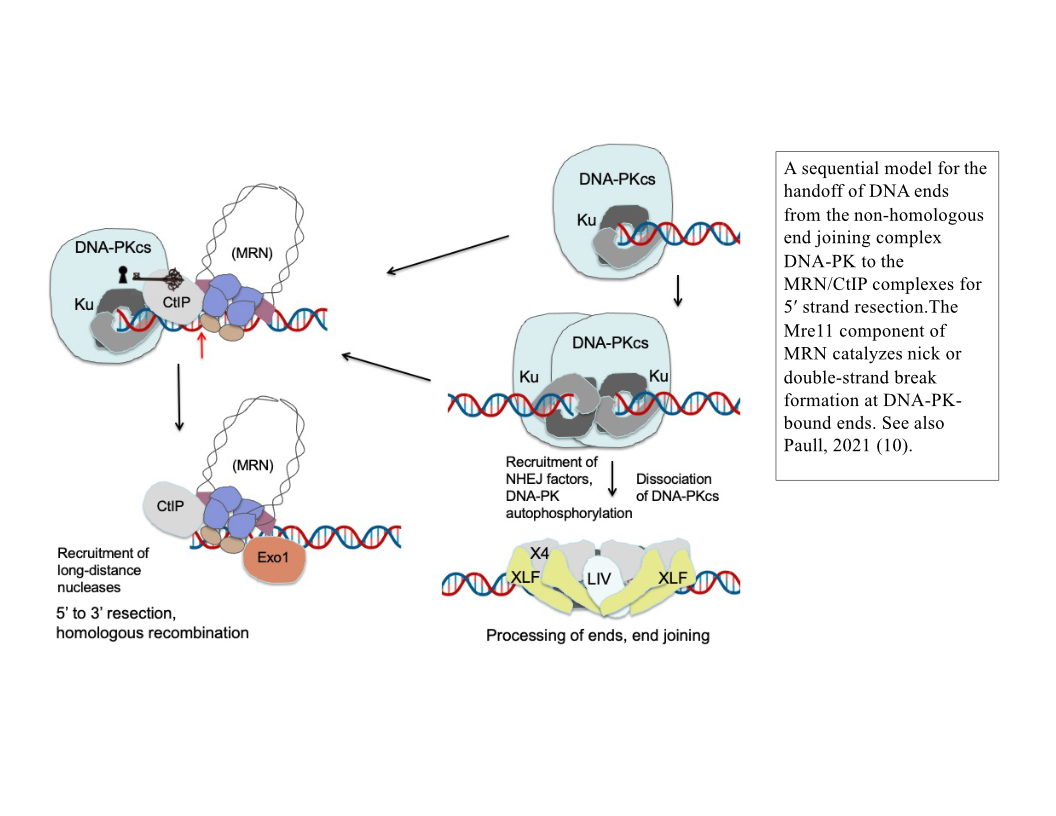
DNA end processing by the MRN complex also requires stimulation by the CtIP protein, a factor specific to eukaryotic cells that is phosphorylated in a cell cycle-dependent manner and is critical for the activation of MRN processing. We have shown that the CtIP protein and its phosphorylation are essential for the activity of Mre11 at DNA-PK-bound sites, both in a reconstituted purified system and in human cells (9).
Transcription-associated DNA lesions
CtIP is a multi-functional protein that also plays important roles at sites of DNA damage that overlap with actively transcribed genes. We have found that loss of CtIP or of Sae2, the ortholog of CtIP in budding yeast, generates R-loops—3-stranded structures containing RNA-DNA hybrids and a displaced DNA strand (11). How CtIP prevents transcription-associated DNA damage is not yet understood, but this is an area of active research in our group. Current studies are investigating how the nuclease activity of CtIP affects the processing of R-loop structures in human cells and what other factors are at R-loops that promote their recognition and removal.
DNA Damage Signaling
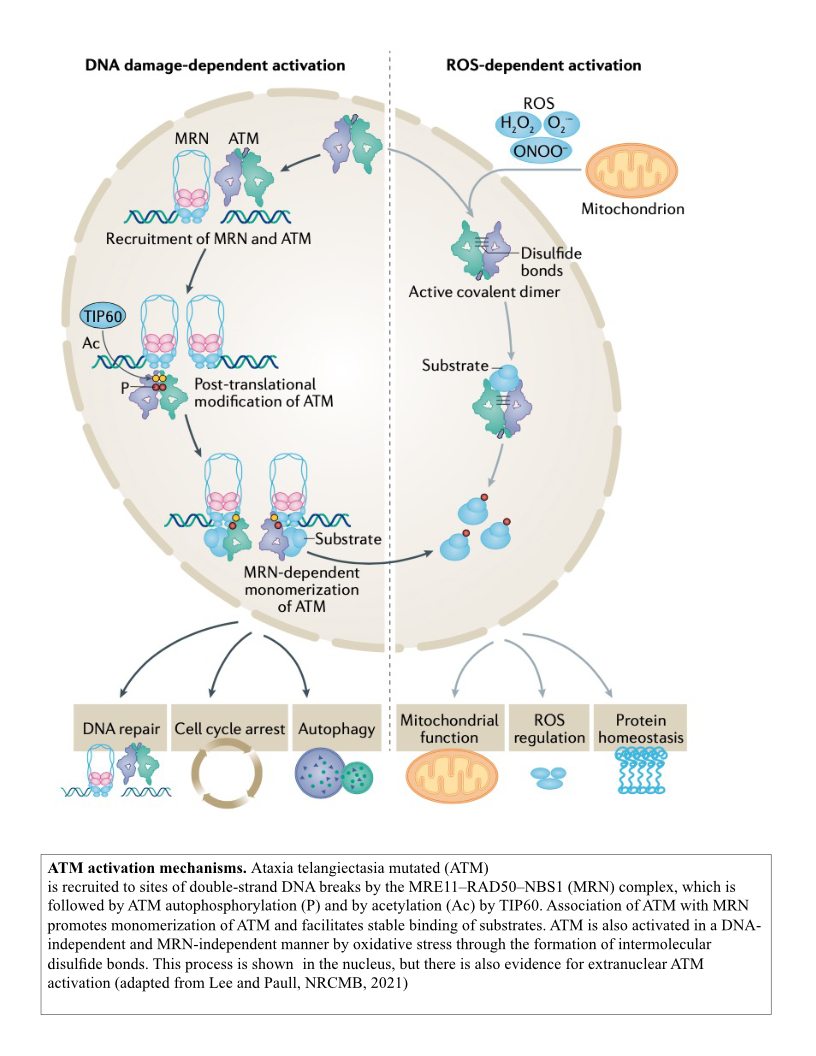
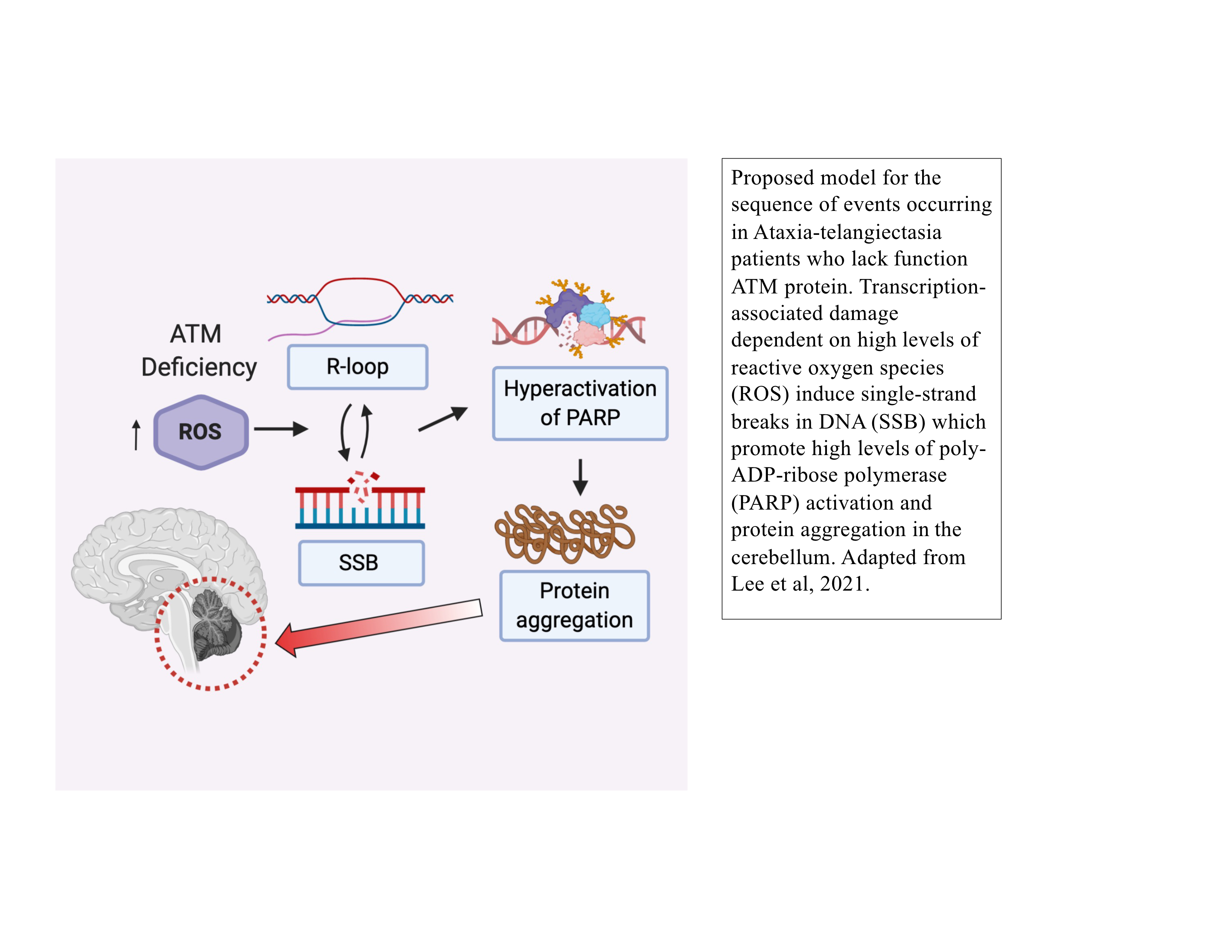
The MRN complex works in concert with the Ataxia-Telangiectasia-Mutated (ATM) protein kinase that phosphorylates many downstream targets responsible for checkpoint activation and DNA damage signaling in eukaryotes. We have previously shown that MRN recruits ATM to broken DNA ends and activates its kinase activity at these sites (12, 13), initiating a cascade of phosphorylation that ultimately affects hundreds of targets in the nucleus. Loss of ATM function in humans is the cause of the genetic disorder Ataxia-Telangiectasia (A-T), which results in a diverse set of clinical symptoms that include childhood-onset cerebellar ataxia, cancer predisposition, and immunodeficiency. Despite many years of study, the molecular basis of neurodegeneration in A-T is not understood. Our recent results examining cerebellum tissue from A-T patients and controls showed that one of the common features of patient brain tissue is protein aggregates—irreversible, detergent-resistant protein assemblies that are found in many common neurodegenerative diseases (14). From experiments in human cells in culture, we know that these aggregates are dependent on the accumulation of transcription-dependent single-strand breaks and that high levels of reactive oxygen species in ATM-deficient cells contributes to these events (15).
Oxidative Stress Responses
We previously discovered that ATM can be activated in an MRN-independent manner through direct oxidation (16). This pathway is important for cellular control of antioxidant functions and for global responses of human cells to reactive oxygen species. Consistent with this role, cells from A-T patients exhibit signs of chronic oxidative stress and some of the tissues that are most affected by ATM loss in mouse models of A-T can be protected from damage through exposure to antioxidants. Importantly, one of the mutations in the ATM gene that causes early-onset ataxia specifically eliminates the ROS-dependent ATM signaling pathway (R3047X), suggesting that loss of this pathway is sufficient to generate the neurodegeneration that occurs in this disease. Current studies are aimed at understanding the molecular basis of the DNA damage that occurs in ATM-deficient cells and how this generates protein aggregates that are associated with and may play a causal role in the neurodegeneration process.
Poly-ADP-ribosylation and DNA damage-induced loss of protein homeostasis
Another very rapid response to DNA damage is the binding and activation of poly-ADP-ribose (PAR) polymerases that catalyze formation of PAR chains on proteins at damage sites. We know from analysis of A-T patient tissues that PAR levels are high in A-T cerebellum tissue, and from experiments in cells in culture we know that polymerization of PAR promotes the protein aggregation events that occur in the absence of ATM function (14). PAR polymers are similar to nucleic acids in charge but can be made and degraded dynamically by PARP enzymes and PAR glycohydrolases. Based on our recent results in patient tissue and human neurons differentiated in culture, we postulate that DNA damage occurring due to high levels of reactive oxygen species in ATM-deficient neurons promotes chronic hyperactivation of PARP enzymes and ultimately results in the irreversible loss of protein function that manifests as protein aggregation (17). Current studies in the lab seek to understand the molecular mechanisms underlying these events and to further understand the changes occurring in human A-T patients that are responsible for the childhood-onset neurodegeneration in this disease.
Poly-ADP-ribosylation and DNA repair outcomes
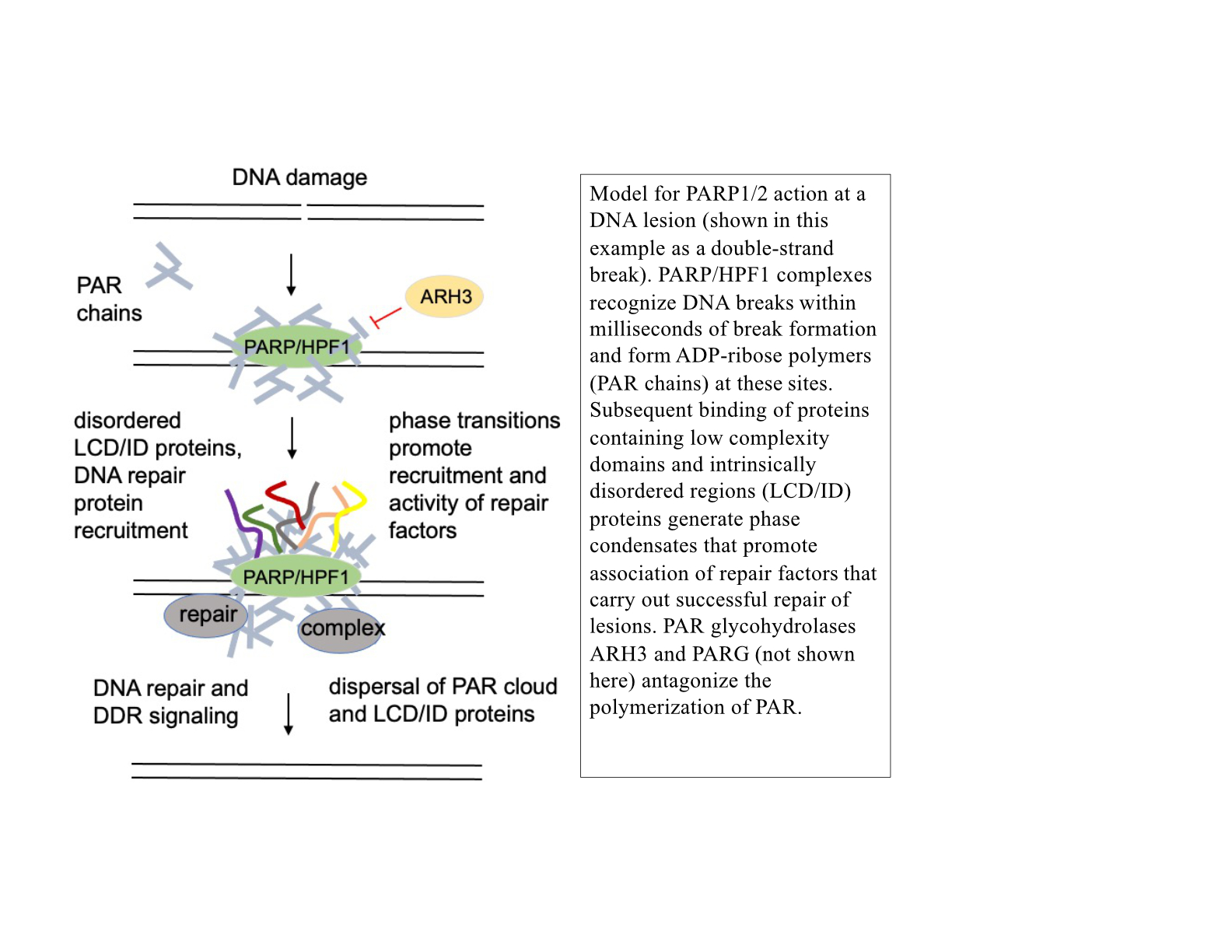
Under normal conditions, PARP activation at sites of DNA lesions promotes successful DNA repair. One of the consequences of this activation is the formation of PAR-dependent phase condensates—protein assemblies seeded by PAR that are dependent on nucleic acid binding domains. Currently we know relatively little about the function of DNA damage-dependent phase condensates and how they function in DNA repair outcomes, but we hypothesize that the condensates are an essential component of DNA repair in living cells. Our studies are focusing on this question, using both in vitro reconstituted systems and human cells to address the molecular basis of these relationships.
Measurements of chaperone clients in human cells
There are significant effects of DNA damage on protein homeostasis but we do not understand these at a molecular level. One way to interrogate misfolded proteins and protein aggregates in cells is by quantifying proteins bound to the ubiquitous protein chaperone HSP70. We have generated a ubiquitination-based system for identifying HSP70 chaperone clients in human cells and have shown that it can be a powerful readout of changes in components in multi-subunit complexes as well as the effects of unstable protein expression (18).
1. T. T. Paull, 20 Years of Mre11 Biology: No End in Sight. Mol. Cell. 71, 419–427 (2018).
2. B. Cannon, J. Kuhnlein, S. H. Yang, A. Cheng, D. Schindler, J. M. Stark, R. Russell, T. T. Paull, Visualization of local DNA unwinding by Mre11/Rad50/Nbs1 using single-molecule FRET. Proceedings of the National Academy of Sciences of the United States of America. 110, 18868–73 (2013).
3. R. A. Deshpande, G. J. Williams, O. Limbo, R. S. Williams, J. Kuhnlein, J.-H. Lee, S. Classen, G. Guenther, P. Russell, J. A. Tainer, T. T. Paull, ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling. EMBO J. 33, 482–500 (2014).
4. R. A. Deshpande, J.-H. Lee, S. Arora, T. T. Paull, Nbs1 Converts the Human Mre11/Rad50 Nuclease Complex into an Endo/Exonuclease Machine Specific for Protein-DNA Adducts. Mol. Cell. 64, 593–606 (2016).
5. R. A. Deshpande, J. H. Lee, T. T. Paull, Rad50 ATPase activity is regulated by DNA ends and requires coordination of both active sites. Nucleic acids research (2017), doi:10.1093/nar/gkx173.
6. J. H. Lee, M. R. Mand, R. A. Deshpande, E. Kinoshita, S. H. Yang, C. Wyman, T. T. Paull, Ataxia Telangiectasia-Mutated (ATM) Kinase Activity Is Regulated by ATP-driven Conformational Changes in the Mre11/Rad50/Nbs1 (MRN) Complex. J Biol Chem. 288, 12840–51 (2013).
7. M. L. Nicolette, K. Lee, Z. Guo, M. Rani, J. M. Chow, S. E. Lee, T. T. Paull, Mre11-Rad50-Xrs2 and Sae2 promote 5’ strand resection of DNA double-strand breaks. Nat Struct Mol Biol. 17, 1478–85 (2010).
8. L. R. Myler, I. F. Gallardo, M. M. Soniat, R. A. Deshpande, X. B. Gonzalez, Y. Kim, T. T. Paull, I. J. Finkelstein, Single-Molecule Imaging Reveals How Mre11-Rad50-Nbs1 Initiates DNA Break Repair. Mol Cell. 67, 891-898 e4 (2017).
9. R. A. Deshpande, L. R. Myler, M. M. Soniat, N. Makharashvili, L. Lee, S. P. Lees-Miller, I. J. Finkelstein, T. T. Paull, DNA-dependent protein kinase promotes DNA end processing by MRN and CtIP. Sci Adv. 6, eaay0922 (2020).
10. T. T. Paull, Reconsidering pathway choice: a sequential model of mammalian DNA double-strand break pathway decisions. Curr Opin Genet Dev. 71, 55–62 (2021).
11. N. Makharashvili, S. Arora, Y. Yin, Q. Fu, X. Wen, J.-H. Lee, C.-H. Kao, J. W. Leung, K. M. Miller, T. T. Paull, Sae2/CtIP prevents R-loop accumulation in eukaryotic cells. Elife. 7 (2018), doi:10.7554/eLife.42733.
12. J. H. Lee, T. T. Paull, Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 304, 93–6 (2004).
13. J.-H. Lee, T. T. Paull, ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 308, 551–554 (2005).
14. J.-H. Lee, S. W. Ryu, N. A. Ender, T. T. Paull, Poly-ADP-ribosylation drives loss of protein homeostasis in ATM and Mre11 deficiency. Mol Cell. 81, 1515-1533.e5 (2021).
15. J.-H. Lee, M. R. Mand, C.-H. Kao, Y. Zhou, S. W. Ryu, A. L. Richards, J. J. Coon, T. T. Paull, ATM directs DNA damage responses and proteostasis via genetically separable pathways. Sci Signal. 11 (2018), doi:10.1126/scisignal.aan5598.
16. Z. Guo, S. Kozlov, M. F. Lavin, M. D. Person, T. T. Paull, ATM activation by oxidative stress. Science. 330, 517–521 (2010).
17. J.-H. Lee, T. T. Paull, Cellular functions of the protein kinase ATM and their relevance to human disease. Nat Rev Mol Cell Biol (2021), doi:10.1038/s41580-021-00394-2.
18. S. W. Ryu, R. Stewart, D. C. Pectol, N. A. Ender, O. Wimalarathne, J.-H. Lee, C. P. Zanini, A. Harvey, J. M. Huibregtse, P. Mueller, T. T. Paull, Proteome-wide identification of HSP70/HSC70 chaperone clients in human cells. PLoS Biol. 18, e3000606 (2020).
-
Publications:
Lee, J-H., Ryu, S.W., Ender, N.A., Paull, T.T. (2021) Poly-ADP-ribosylation drives loss of protein homeostasis in ATM and Mre11 deficiency. Molecular Cell 81:1515-1533
Somyajit K., Spies J., Coscia F., Kirik U., Rask M.B., Lee J.H., Neelsen K.J., Mund A., Jensen L.J., Paull T.T., Mann M., Lukas J. (2021) Homology-directed repair protects the replicating genome from metabolic assaults. Developmental Cell 6(4):461-477.e7.
Milanovic, M., Houghton, L.M., Menolfi, D., Lee, J-H., Yamamoto, K., Li, Y., Lee, B.J., Xu, J., Estes, V.M., Wang, D., Mckinnon, P.J., Paull, T.T., Zha, S. The Cancer-Associated ATM R3008H Mutation Reveals the Link between ATM Activation and Its Exchange. (2021) Cancer Res. 81(2):426–437. PMCID: PMC8137556
Cirotti C., Rizza S., Giglio P., Poerio N., Allega M.F., Claps G., Pecorari C., Lee J., Benassi B., Barilà D., Robert C., Stamler J.S., Cecconi F., Fraziano M., Paull T.T., and Filomeni, G. (2020) Redox activation of ATM enhances GSNOR translation to sustain mitophagy and tolerance to oxidative stress. EMBO Rep doi/10.15252/embr.202050500.
Ryu, S.W., Stewart, R., Pectol, D.C., Ender, N., Wimalarathne, O., Lee, J-H., Zanini, C.P., Harvey, A., Huibregtse, J.M., Mueller, P., and Paull, T.T. (2020) Proteome-wide identification of HSP70/HSC70 Chaperone Clients in Human Cells, PloS Biology 18(7): e3000606. https://doi.org/10.1371/journal.pbio.3000606.
Paiano, J., Wu, W., Yamada, S., Sciascia, N., Callen, E., Paola, Cotrim A., Deshpande, R.A., Maman, Y., Day, A., Paull, T.T., Nussenzweig, A. ATM and PRDM9 regulate SPO11-bound recombination intermediates during meiosis. (2020) Nat Commun. 2020 Feb 12;11(1):857.
Kao, C.-H., Ryu, S., Kim, M.J., Wen, X., Wimalarathne, O., and Paull, T.T. (2020). Growth-regulated Hsp70 phosphorylation regulates stress responses and prion maintenance. Mol. Cell. Biol. MCB.00628-19.
Deshpande, R.A., Myler, L.R., Soniat, M.M., Makharashvili, N., Lee, L., Lees-Miller, S.P., Finkelstein, I.J., Paull, T.T. DNA-PKcs promotes DNA end processing by MRN and CtIP. (2020) Science Advances 6(2):eaay0922.
Kim, J.J., Lee S.Y., Gong, F., Battenhouse, A.M., Boutz, D.R., Bashyal, A., Refvik, S.T., Chang, C-M., Xhemalce, B., Paull, T.T., Brodbelt, J.S., Marcotte, E.M., and Miller, K.M. (2019) Systematic Bromodomain Protein Screens Identify Homologous Recombination and R-Loop Suppression Pathways Involved in Genome Integrity. Genes & Development 33(23–24):1751–74. https://doi.org/10.1101/gad.331231.119.
Soniat M.M., Myler L.R., Kuo H-C., Paull T.T., Finkelstein I.J. RPA Phosphorylation Inhibits DNA Resection. (2019) Molecular Cell 75, 145-153.
Myler L.R., Soniat M.M., Zhang X., Deshpande R.A., Paull T.T., Finkelstein IJ. Purification and Biophysical Characterization of the Mre11-Rad50-Nbs1 Complex. (2019) Methods Mol Biol.2004:269–287.
Makharashvili, N., Arora, S., Yin, Y., Fu, Q., Wen, S., Lee, J.-H., Kao, C.-H., Leung, J.W.C., Miller, K.M., Paull, T.T. Sae2/CtIP Prevents R-Loop Accumulation in Eukaryotic Cells. eLife 7 (2018). https://doi.org/10.7554/eLife.42733.
Johnson, T.E., Lee, J.-H., Myler, L.R., Zhou, Y., Mosley, T.J., Yang, S.-H., Uprety, N., Kim, J., and Paull, T.T. (2018). Homeodomain Proteins Directly Regulate ATM Kinase Activity. Cell Reports 24, 1471–1483.
Lee, J.-H., Mand, M.R., Kao, C.-H., Zhou, Y., Ryu, S.W., Richards, A.L., Coon, J.J., and Paull, T.T. (2018). ATM directs DNA damage responses and proteostasis via genetically separable pathways. Science Signaling 11(512). pii: eaan5598. doi: 10.1126/scisignal.aan5598.
Hung, P.J., Johnson, B., Chen, B.-R., Byrum, A.K., Bredemeyer, A.L., Yewdell, W.T., Johnson, T.E., Lee, B.J., Deivasigamani, S., Hindi, I., Amatya, P., Gross, M.L., Paull, T.T., Pisapia, D.J., Chaudhuri, J., Petrini, J.H., Mosammaparast, N., Amarasinghe, G.K., Zha, S., Tyler, J.K., and Sleckman, B.P. (2018). MRI Is a DNA Damage Response Adaptor during Classical Non-homologous End Joining. Molecular Cell 71, 332-342.e8.
Zhang, Y., Lee, J.-H., Paull, T.T., Gehrke, S., D’Alessandro, A., Dou, Q., Gladyshev, V.N., Schroeder, E.A., Steyl, S.K., Christian, B.E., and Shadel, G.S. (2018). Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Science Signaling 11(538). pii: eaaq0702. doi: 10.1126/scisignal.aaq0702.
Hewitt, S.L., Wong, J.B., Lee, J.H., Nishana, M., Chen, H., Coussens, M., Arnal, S.M., Blumenberg, L.M., Roth, D.B., Paull, T.T., and Skok, J.A. (2017). The Conserved ATM Kinase RAG2-S365 Phosphorylation Site Limits Cleavage Events in Individual Cells Independent of Any Repair Defect. Cell Reports 21, 979-993.
Arora, S., Deshpande, R.A., Budd, M., Campbell, J., Revere, A., Zhang, X., Schmidt, K.H., and Paull, T.T. (2017) Genetic separation of Sae2 nuclease activity from Mre11 nuclease functions in budding yeast. Molecular and Cellular Biology 37: e00156-17.
Myler, L.R., Gallardo, I.F., Deshpande, R.A., Gonzalez, X.B., Kim, Y., Paull, T.T., and Finkelstein, I.J. (2017). Single-molecule imaging reveals how Mre11-Rad50-Nbs1 initiates DNA break repair. Molecular Cell 67, 891-898.
Deshpande, R., Lee, J.-H., and Paull, T.T. (2017). Rad50 ATPase activity is regulated by DNA ends and requires coordination of both active sites. Nucleic Acids Research 45, 5255-5268.
Zhou, Y., Lee, J.H., Jiang, W., Crowe, J.L., Zha, S., and Paull, T.T. (2017). Regulation of the DNA Damage Response by DNA-PKcs Inhibitory Phosphorylation of ATM. Molecular Cell 65, 91-104.
Deshpande, R.A., Lee, J.H., Arora, S., and Paull, T.T. (2016). Nbs1 Converts the Human Mre11/Rad50 Nuclease Complex into an Endo/Exonuclease Machine Specific for Protein-DNA Adducts. Molecular Cell 64, 593-606.
Hoa, N.N., Shimizu, T., Zhou, Z.W., Wang, Z.Q., Deshpande, R.A., Paull, T.T., Akter, S., Tsuda, M., Furuta, R., Tsutsui, K., et al. (2016). Mre11 Is Essential for the Removal of Lethal Topoisomerase 2 Covalent Cleavage Complexes. Molecular Cell 64, 580-592.
Myler, L.R., Gallardo, I.F., Zhou, Y., Gong, F., Yang, S.H., Wold, M.S., Miller, K.M., Paull, T.T.*, and Finkelstein, I.J*. (2016). Single-molecule imaging reveals the mechanism of Exo1 regulation by single-stranded DNA binding proteins. Proceedings of the National Academy of Sciences of the United States of America 113, E1170-1179. * co-contributing
Broderick, R., Nieminuszczy, J., Baddock, H.T., Deshpande, R.A., Gileadi, O., Paull, T.T., McHugh, P.J., and Niedzwiedz, W. (2016). EXD2 promotes homologous recombination by facilitating DNA end resection. Nature Cell Biol. 18, 271-280.
Zhang, J., Tripathi, D., Jing, J., Alexander, A., Kim, J., Powell, R., Dere, R., Tait-Mulder, J., Lee, J-H., Paull, T.T., Pandita, R.K., Charaka, V., Pandita, T., Kastan, M., and Walker, C. (2015). ATM Functions at the Peroxisome to Induce Pexophagy in Response to ROS. Nature Cell Biol. 17:1259-1269.
Sarangi, P., Steinacher, R., Altmannova, V., Fu, Q., Paull, T.T., Krejci, L., Whitby, M.C., and Zhao, X. (2015). Sumoylation influences DNA break repair partly by increasing the solubility of a conserved end resection protein. PLoS Genetics 11, e1004899.
Hoa, N.N., Kobayashi, J., Omura, M., Hirakawa, M., Yang, S.H., Komatsu, K., Paull, T.T., Takeda, S., and Sasanuma, H. (2015). BRCA1 and CtIP Are Both Required to Recruit Dna2 at Double-Strand Breaks in Homologous Recombination. PloS One 10, e0124495.
Zhou, Y., and Paull, T.T. (2015). Direct measurement of single-stranded DNA intermediates in mammalian cells by quantitative polymerase chain reaction. Anal Biochem 479, 48-50.
Parameswaran, B., Chiang, H.C., Lu, Y., Coates, J., Deng, C.X., Baer, R., Lin, H.K., Li, R., Paull, T.T., and Hu, Y. (2015). Damage-induced BRCA1 phosphorylation by Chk2 contributes to the timing of end resection. Cell Cycle 14, 437-448.
Makharashvili, N., Tubbs, A.T., Yang, S.H., Wang, H., Barton, O., Zhou, Y., Deshpande, R.A., Lobrich, M., Sleckman, B.P., Wu, X., and Paull, T.T. (2014). Catalytic and noncatalytic roles of the CtIP endonuclease in double-strand break end resection. Molecular Cell 54(6): 1022-1023.
Lee, J.-H., Guo, Z., Myler, L.R., Zheng, S., and Paull, T.T. (2014). Direct activation of ATM by resveratrol under oxidizing conditions. PloS ONE 9(6):e97969.
Deshpande, R., Williams, G.J., Limbo, O., Williams, R.S., Kuhnlein, J., Lee, J.-H., Classen, S., Guenther, G., Russell, P., Tainer, J.A., and Paull, T.T. (2014). ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling. EMBO J.33(5):482-500.
Zhou, Y., and Paull, T.T. (2014). Quantitation of DNA double-strand break resection intermediates in human cells. Nucleic Acids Research 42(3):e19.
Barton, O., Naumann, S.C., Diemer-Biehs, R., Kunzel, J., Steinlage, M., Conrad, S., Makharashvili, N., Wang, J., Feng, L., Lopez, B.S., Paull, T.T., Chen, J., Jeggo, P.A. and Lobrich, M. (2014). Polo-like kinase 3 regulates CtIP during DNA double-strand break repair in G1. The Journal of Cell Biology 206, 877–894.
Fu, Q., Chow, J, Bernstein, K.A., Makharashvili, N., Arora, S., Lee, C-F., Person, M., Rothstein, R., and Paull, T.T. (2013) Phosphorylation-regulated transitions in oligomeric state control the activity of the Sae2 DNA repair enzyme. Mol. Cell. Biol. 34(5):778-793.
Cannon, B., Kuhnlein, J., Yang, S.H., Cheng, A., Schindler, D., Stark, J.M., Russell, R., and Paull, T.T. (2013). Visualization of local DNA unwinding by Mre11-Rad50-Nbs1 using single-molecule FRET. Proceedings of the National Academy of Sciences of the United States of America 110(47):18868-73.
Zhou, Y., and Paull, T.T. (2013). DNA-dependent Protein Kinase regulates DNA end resection in concert with Mre11-Rad50-Nbs1 (MRN) and Ataxia-Telangiectasia-Mutated (ATM). J. Biol. Chem. 288(52):37112-25.
Bowen, C., Ju, J.H., Lee, J-H., Paull, T.T., and Gelmann, E.P. (2013). Functional Activation of ATM by the Prostate Cancer Suppressor NKX3.1. Cell Reports 4, 516-529.
Lee, J-H., Mand, M.R., Deshpande, R.A., Kinoshita, E., Yang, S-H., Wyman, C., and Paull, T.T. (2013). Ataxia Telangiectasia-Mutated (ATM) Kinase Activity Is Regulated by ATP-driven Conformational Changes in the Mre11/Rad50/Nbs1 (MRN) Complex. J Biol Chem 288, 12840-12851.
Yang, S-H., Zhou, R., Campbell, J., Chen, J., Ha, T., and Paull, T.T. (2013) The SOSS1 single-stranded DNA binding complex promotes DNA end resection in concert with Exo1. EMBO J 32(1):126-39.
Daniel, J.A., Pellegrini, M., Lee, B.S., Guo, Z., Filsuf, D., Belkina, N.V., You, Z., Paull, T.T., Sleckman, B.P., Feigenbaum, L., Nussenzweig, A. (2012) Loss of ATM kinase activity leads to embryonic lethality in mice. J Cell Biol 198(3): 295-304
Gatei, M., Jakob, B., Chen, P., Kijas, A.W., Becherel, O.J., Gueven, N., Birrell, G., Lee, J.H., Paull, T.T., Lerenthal, Y., Fazry, S. Taucher-Scholz, G., Kalb, R., Schindler, D., Waltes, R., Dork, T., and Lavin, M. F. (2011). ATM protein-dependent phosphorylation of Rad50 protein regulates DNA repair and cell cycle control. J Biol Chem 286, 31542-31556.
Della-Maria, J., Zhou, Y., Tsai, M.S., Kuhnlein, J., Carney, J., Paull, T.T., Tomkinson A (2011) hMre11/hRad50/Nbs1 and DNA ligase III{alpha}/XRCC1 act together in an alternative non-homologous end joining pathway. J Biol Chem. 286(39): 33845-33853.
Guo, Z., Deshpande, R., and Paull, T.T. (2010). ATM activation in the presence of oxidative stress. Cell Cycle 9(24):4805-11.
Nicolette, M.L., Lee, K., Guo, Z., Rani, M., Chow, J.M., Lee, S.E. and Paull, T.T. (2010) A direct role for Mre11/Rad50/Xrs2 and Sae2 in 5’ strand resection of DNA double-strand breaks. Nature Structural and Molecular Biology 17(12):1478-85.
Guo, Z., Kozlov, S., Lavin, M.F., Person, M.D., and Paull, T.T. (2010) ATM Activation by Oxidative Stress. Science 330:517-521.
Shim, E.Y., Chung, W.H., Nicolette, M.L., Zhang, Y., Davis, M., Zhu, Z., Paull, T.T., Ira, G., and Lee, S.E. (2010). Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J 29, 3370-3380.
Demogines, A., East, A.M., Lee, J.H., Grossman, S.R., Sabeti, P.C., Paull, T.T., and Sawyer, S.S. (2010) Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes. PLOS Genetics 6(10):e1001169.
Lee, J.H., Goodarzi, A.A., Jeggo, P.A., and Paull, T.T. (2010) 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 29:574-585.
Hopkins, B.B., and Paull, T.T. (2008) The P. furiosus Mre11/Rad50 complex promotes 5’ strand resection at a DNA double-strand break. Cell 135: 250-260.
Daniel, J.A., Pellegrini, M., Lee, J-H., Paull, T.T., Feigenbaum, L., and Nussenzweig, A. (2008) Multiple autophosphorylation sites are dispensable for murine ATM activation in vivo. J. Cell Biol. 183:777-783.
Richard D.J., Bolderson, E., Cubeddu, L., Wadsworth, R.I.M., Savage, K., Sharma, G.G., Nicolette, M.L., Tsvetanov, S., McIlwraith, M.J., Pandita, R., Takeda, S., Hay, R.T., Gautier, J., West, S.C., Paull, T.T., Pandita, T.K., , White, M.F., and Khanna, K.K. (2008) Evolutionary conserved single stranded DNA binding protein ‘hSSB1’ is critical for genomic stability. Nature 453: 677-681.
Dupre, A., Boyer-Chatenet, L., Sattler, R.M., Modi, A.P., Lee, J.H., Nicolette, M.L., Kopelovich, L., Jasin, M., Baer, R., Paull, T.T., and Gautier, J. (2008). A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat. Chem. Biol. 4, 119-125.
Lengsfeld, B.M., Rattray, A.J., Bhaskara, V., Ghirlando, R., and Paull, T.T. (2007). Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Molecular Cell 28: 638-651.
Bhaskara, V., Dupre, A., Lengsfeld, B., Hopkins, B. B., Chan, A., Lee, J. H., Zhang, X., Gautier, J., Zakian, V. A., and Paull, T. T. (2007). Rad50 Adenylate Kinase Activity Regulates DNA Tethering by Mre11/Rad50 complexes. Mol. Cell 25: 647-661.
Hunt, C.R., Pandita, R.K., Laszlo, A., Higashikubo, R., Agarwal, M., Kitamura, T., Gupta, A., Rief, N., Horikoshi, N., Baskaran, R., Lee, J.H., Lobrich, M., Paull, T.T., Roti Roti, J.L., and Pandita, T.K. (2007). Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res 67: 3010-3017.
Lou, Z., Minter-Dykhouse, K., Franco, S., Gostissa, M., Rivera, M. A., Celeste, A., Manis, J. P., van Deursen, J., Nussenzweig, A., Paull, T. T., Alt, F. W., and Chen, J. (2006). MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell 21: 187-200.
Zhang, X. and Paull, T.T. (2005) The Mre11/Rad50/Xrs2 complex and non-homologous end-joining of incompatible ends in S. cerevisiae. DNA Repair 4: 1281-1294.
Gupta, A., Sharma, G.G., Young, C.S., Agarwal, M., Smith, E.R., Paull, T.T., Lucchesi, J.C., Khanna, K.K., Ludwig, T. and Pandita, T.K. (2005) Involvement of Human MOF in ATM Function. Mol. Cell. Biol. 25: 5292-5305.
Lee, J.H. and Paull, T.T. (2005) ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science, 308: 551-554.
Costanzo, V., Paull, T.T., Gottesman, M., and Gautier, J. (2004). Mre11 assembles linear DNA fragments into DNA damage signaling complexes. PLoS Biol 2, E110
Lee, J.-H. and Paull, T.T. (2004). The Mre11/Rad50/Nbs1 complex directly promotes ATM kinase activity. Science 304: 93-96.
Moncalian, G., Lengsfeld, B., Bhaskara, V., Hopfner, K.P., Karcher, A., Alden, E., Tainer, J.A. and Paull, T.T. (2004) The rad50 signature motif: essential to ATP binding and biological function. J Mol. Biol. 335: 937-951.
Lee, J.-H., Ghirlando, R., Bhaskara, V., Hoffmeyer, M.R., Gu, J. and Paull, T.T. (2003) Regulation of Mre11/Rad50 by Nbs1: effects on nucleotide-dependent DNA binding and association with ATLD mutant complexes. J. Biol. Chem. 278: 45171-45181.
-
BIO391
-














